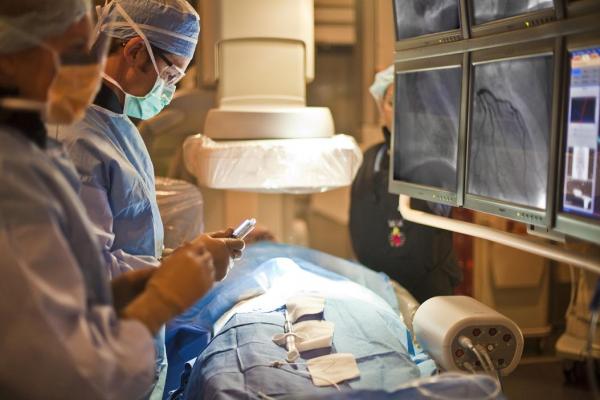
May 11, 2012 — Modern cardiac catheterization laboratories bear scant resemblance to the cath labs of a decade ago. An updated consensus statement released this week offers physicians guidance on how to excel in this new diagnostic and therapeutic milieu, with specific recommendations on setting up, operating and maintaining the highest standards of quality in a contemporary cardiac cath lab.
The consensus statement was developed by a panel of the American College of Cardiology Foundation (ACCF) and the Society for Cardiovascular Angiography and Interventions (SCAI), in collaboration with the Society of Thoracic Surgeons and the Society for Vascular Medicine. It updates a similar document released in 2001.
“There have been a lot of changes since 2001, some of them dramatic,” said Thomas M. Bashore, M.D., writing committee chair and professor of medicine and clinical chief of cardiology at Duke University Medical Center, Durham, N.C. “This document sets the stage for what’s really happening in cath labs today and updates everyone on accepted quality standards and best practices.”
Among the changes has been a shift in focus from diagnostic tests to catheter-based therapies, from coronary disease alone to include the treatment of valvular heart disease, congenital defects of the heart and arterial disease in the legs, brain and other organs. An increasing number of medical centers are developing hybrid cath labs that combine all the features of a surgical suite with those of a cath lab. And pediatric cath labs—now devoted almost exclusively to therapy—apply minimally invasive catheter techniques to congenital disease that once required major heart surgery, with procedures now being performed on unborn fetuses and newborns, as well as older children.
The new consensus statement addresses the features of modern cath labs, including:
- Cardiac catheterization laboratories without on-site cardiac surgical backup: Today, up to one-third of angiograms and percutaneous coronary interventions (PCIs) are performed in cath labs without on-site surgery. The consensus document states the risk of major complications with most invasive coronary procedures is very low and proposes requirements for establishing off-site surgical backup while lifting many prior restrictions on the types of patients eligible for such procedures.
- PCI for heart attack patients in hospitals without on-site cardiac surgical backup: The authors stress that partnership with a major medical center with cardiac surgery capability is mandatory, including the establishment of a written plan for transfer should complications arise. The document withholds endorsement of programs that offer PCI for heart attack only during daytime business hours (no nights or weekends).
- Minimum case volumes for diagnostic cardiac catheterization: The authors found no data to support the use of minimum case volumes as an indicator of physician quality in performing diagnostic cardiac catheterization. Instead, they state that an effective quality assurance program is the key to ensuring that cardiac catheterization studies are appropriate, and performed and interpreted correctly. A related document is in development and will address minimal case volume for physicians who perform PCI procedures.
- Quality assurance/quality improvement programs: The new standards document defines essential elements of a quality assurance/quality improvement program, including participation in a national clinical database, tracking of specific quality indicators, benchmarking against peers, and monitoring of procedure appropriateness and quality.
- Hybrid cath labs: The authors review in detail the role of the hybrid cath lab, including the types of procedures suitable for a hybrid lab and special considerations in designing, building and equipping the lab.
- Training requirements: The document outlines the current training requirements for physicians who perform invasive and interventional procedures in the coronary and peripheral arteries, and for the treatment of structural heart disease.
- Pre-procedural preparation: The authors recommend steps to protect kidney function during invasive procedures, including ensuring adequate hydration, shortening the time patients are instructed to go without drinking before the procedure and linking the maximum volume of contrast media to pre-catheterization kidney function tests. The document also suggests a routine protime is no longer required before the catheterization unless there is a reason to suspect an abnormality.
- Imaging: The authors also summarize major changes in X-ray imaging, with the adoption of digital image acquisition, display and storage.
- Radiation safety: The document outlines techniques to reduce radiation exposure to cath lab patients and staff.
- Treatment of infants and children: The authors explore in depth special concerns involving the pediatric cardiac catheterization laboratory.
Consensus documents serve many purposes, but the most important is to define high-quality clinical practice, Bashore said.
“We don’t always have randomized clinical trials to rely on,” he said. “The purpose of this document is to define a practice standard for how you set up and run a cath lab, the people in the cath lab, the quality assurance programs—all the things cath lab teams should be thinking about.”
The document can be accessed at www.cardiosource.org and www.SCAI.org. It will also be published in the June 5, 2012, issue of the Journal of the American College of Cardiology and in a forthcoming issue of Catheterization and Cardiovascular Interventions.


 November 14, 2025
November 14, 2025 









