The latest trends and newest cardiac rhythm management technology were highlighted during the biggest electrophysiology (EP) meeting in the world, Heart Rhythm Society (HRS) 2012 in May, held in Boston. Several new advances were showcased that may have a major impact on the future of EP.
Subcutaneous ICD
Device-wise, the Cameron Health subcutaneous implantable cardioverter defibrillator (S-ICD) was the star of the show at this year’s HRS. It is widely expected to revolutionize ICD implantation and greatly expand its implant base because of the greatly simplified procedure and low complication rate. The Cameron booth was packed with interested EPs and several sessions discussed the device, including a late-breaking abstract on the U.S. pivotal trial.
On April 26, 2012, the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel recommended approval of the system, which many experts say might be cleared by FDA this summer. If so, it will be the first implantable EP therapy device to use a subcutaneous lead system. This eliminates the need for venous access, venous leads and endocardial lead securement. The lead implant procedure uses three small incisions and the lead is pulled through just under the skin under direct visualization, eliminating the need for fluoroscopy and the EP to wear lead.
Boston Scientific is in negotiations to purchase Cameron Health and the transaction is expected to take place later this year.
To view a video presentation of Cameron Health’s U.S. pivotal trial data at HRS and to see how the system in implanted, click here.
Ripple and FIRM Mapping of Cardiac Arrhythmias
Researchers are developing new ways to better visualize arrhythmic electrical activity in the heart to better guide catheter ablations. The current technology uses mapping catheters to show the electrical activity at hundreds of points inside the walls of the heart. The EP then needs to annotate these dots, plotted on a 3-D rendering of the chamber of the heart, to show what they think is the flow of electrical activity in the tissue. The process can be subjective and requires the physician to take their best guess on the electrical activity in the areas between the dots.
Two new visualization methods were explained in sessions at HRS 2012, including ripple mapping and focal impulse and rotor modulation (FIRM). Both methods are supposed to speed the mapping process and use complex computer algorithms to process data into maps that offer a clearer picture of electrical activity.
Ripple mapping uses the same 3-D image of the heart as conventional electromapping, but each point is illustrated as a toothpick shaped bar extending from the heart. These points use a color-coding system and the height of the bars to illustrate electrical activity in millivolts. The map can be put into a cine loop to show the flow and intensity of the electrical signal during each heartbeat, each bar moving and lighting up with color to better visualize intensity.
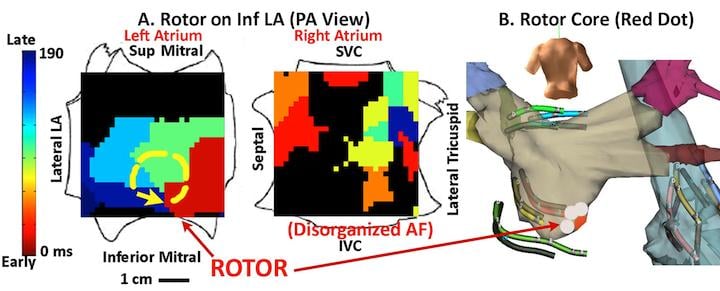
The FIRM electromapping technique can better visualize the rotor (similar to the eye of a hurricane) around which electrical activity, called initiating spirals, rotates in atrial fibrillation (AFib) patients. Created using a 64-pole basket mapping catheter, the map is a flatted electrogram view of the entire chamber of the heart. It is put into motion in a cine loop and uses color to indicate electrical intensity. The flattened map more easily visualizes the rotor location.
Using this map, an ablation catheter can be placed on the rotor center and results can be seen immediately. By using this targeted technique, in some cases a single ablation can terminate the AFib and restore normal rhythm. In conventional treatment, up to six hours of ablation over a large area of the heart is needed to achieve a similar result.
The technique was developed by Sanjiv M. Narayan, M.D., Ph.D., FHRS, associate professor of medicine in residence, co-director, EP fellowship training program, director, VA EP program, University of California San Diego School of Medicine, VA Medical Center. He said it has enabled shorter procedures to acutely terminate or slow AFib. Some of the procedures took less than two hours (most of the time spent creating the map), with ablation time taking less than 10 minutes. If the technique is proven viable in further studies, it will likely change how AFib catheter ablations are performed and could significantly improve outcomes and procedural efficiency.
For more detail on the FIRM technique, click here.
Noninvasive Electromapping
CardioInsight showed a technology that noninvasively electromaps the heart to diagnose arrhythmias, pre-plan ablation procedures and for better optimization of CRT (cardiac resynchronization therapy) lead implants and pacemaker adjustments. The system uses a 256-lead ECG (electrocardiogram) vest, which the patient wears during a computed tomography (CT) scan. A CT 3-D reconstruction of the heart is used as a model on which electrical activity is superimposed from the ECG vest. The imaging is created in one heartbeat and produces a colored electromap similar to invasive catheter-based mapping systems.
The fast electromapping technique shows the entire heart, while catheter mapping is time-consuming and only images one chamber.
The system gained European market clearance this spring and is FDA 510(k) pending.
Automated CRT Optimization in Heart Failure Patients
Sorin Group released several new EP devices into the U.S market at HRS 2012, and the company plans to introduce new technology to complete against established U.S. market leaders. One of its most innovative technologies demonstrated at the show was SonR, which uses a pressure sensor in the tip of the pacing lead to automatically optimize CRT-D therapy. This compensates for cardiac remodeling in heart failure patients. SonR is available in Europe, and Sorin is in negotiations with the FDA to start an investigational device exemption (IDE) trial in the United States.
The company said SonR has a high reorder rate among European EPs, and these doctors report many patients who were unresponsive to previous therapy respond well with the automated optimization.
Disposable Holter Monitors
A new trend emerging in EP monitoring is the use of disposable Holter monitors. The biggest advantages are the low cost and ease of use. Instead of Holter systems that require a $5,000-$10,000 investment, the disposable monitors cost about $50 each, do not require the placement of leads and are mailed in rather than requiring patients to return the device in person.
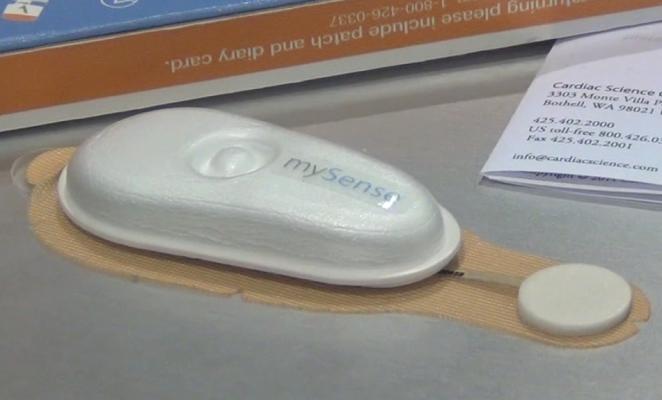
Cardiac Science unveiled and launched its disposable mySense 24-hour Holter monitor system, and iRhythm’s Zio Patch system was highlighted in a study presentation by Scripps Health. Both devices are designed for patients presenting with syncope, chest pain or other possible arrhythmic events. The lightweight Holters are smaller than a candy bar. They use a peel and stick pad to adhere to the patient’s chest. After monitoring, the patient mails it in using a small postage-paid box.
The 285 patient study conducted at Scripps Health found the Zio Patch helped 59 percent of emergency room patients avoid unnecessary follow-up care.
"The availability of this new heart monitor is exciting as it improves patient care. The patch is applied, and when recording is done, the patient simply drops it in the envelope and returns it through the mail – it's like the Netflix of heart care," said electrophysiologist Steven Higgins, M.D., chairman of the department of cardiology at Scripps Memorial Hospital in La Jolla, Calif., and a lead investigator. "Because they are infrequent, heart rhythm problems are often difficult to diagnose, even though they can be quite serious. The Zio Patch is a new digital advance that will allow us to better diagnose challenging cases so we can provide our patients the best care."
Study Qualifies Malfunction Rate of Riata Leads
In November 2011, St. Jude Medical began a recall of its Riata and Riata ST ICD leads because of high rates of externalized conductors, which could cause failure to deliver appropriate therapy or the delivery of inappropriate shocks. The FDA upgraded the response to a Class I recall in December. An estimated 79,000 Riata and Riata ST family of silicone leads remain active in patients in the United States. The company stopped making both leads in December 2010, but physicians want to know what the failure rate of these leads are and how to best manage patients with the implants.
During HRS 2012, there were several abstracts presented on patient safety issues associated with the Riata leads. One session, highlighted as late-breaking abstract, featured the first multi-center study of Riata lead failure. The seven-center, retrospective study included 1,081 patients who had Riata leads implanted between 2002 and 2010. Presenting the results of "Independent Multicenter Study of Riata and Riata ST ICD Leads," Raed H. Abdelhadi, M.D., FACC, cardiologist, electrophysiologist researcher, Minneapolis Heart Institute Foundation, said the Riata leads had a 1.9 percent failure rate. The Riata ST lead had a failure rate of 0.47 and the Quattro Secure leads 0.43.
To watch a video of this HRS presentation, click here.
Car Seats for Heart Patients
The Ford Motor Company displayed a sport utility vehicle on the show floor to draw attention to its research efforts to incorporate ECG sensors directly into the seats of the car. Prof. Dr. Pim van der Jagt, managing director, Ford Research and Advanced Engineering Center, Europe, asked electrophysiologists their opinions on the utility of such a system. It could monitor cardiac patients or act as an early detection system for Ford owners who develop an arrhythmia and were not even aware of it.
New Technology on the Show Floor
• Biosense Webster showed its new ThermoCool-SF (surround flow) ablation catheter. It offers two improvements over the previous ThermoCool. The new system has 56 holes instead of six for better tip cooling. The second advance is an additional 40-90 mm of the catheter length now being visualized on the Carto3 navigation system. Previously only the tip was shown, and the orientation of the catheter was not always evident. The extended catheter visualization also shows how it is braced in the heart chamber to enable more efficient ablations.
• St. Jude Medical showed the industry’s first quadripolar pacing system, the Unify Quadra CRT-D and Quartet Left Ventricular Quadripolar Pacing Lead. It combines multiple pacing configurations that enable physicians to optimize the system at implant and follow-up, as well as better manage common pacing complications without having to surgically reposition the lead. The additional pacing options allow easier solutions when dealing with issues such as infarcts.
• The FDA recently expanded Medtronic’s cardiac resynchronization therapy with implantable cardioverter defibrillator (CRT-D) indication for mild Class II heart failure patients. This follows a similar expanded indication for Boston Scientific’s CRT-D systems. It allows earlier device therapy to prevent patients from declining into Class III and IV heart failure and helps reduce hospitalizations.
• Medtronic’s Revo MRI-safe pacemaker and several other MRI-safe EP devices being developed in Europe gained interest. Many patients requiring implantable EP devices have other health aliments requiring medical imaging. MRI (magnetic resonance imaging) offers a radiation-free imaging option, and these MRI-safe devices will allow expansion of its use.
To watch a video version of this story from the HRS show floor, click here.




 January 15, 2026
January 15, 2026 









