Percutaneous ventricular assist (pVAD) devices offer more hemodynamic support than the 40-year-old gold standard of intra-aortic balloon pumps (IABPs), but their cost and complexity has limited their use to all but the sickest patients. However, the quest for improving patient outcomes and new applications of pVADs for right ventricular support and heart failure indications continues to expand interest in these devices. Several of these new technologies were highlighted in sessions during the 2013 Transcatheter Cardiovascular Therapeutics (TCT) last fall.
Each year acute percutaneous mechanical circulatory support is used in an estimated 230,000, said Ulrich Jorde, M.D., medical director, mechanical circulatory support program at New York-Presbyterian Hospital/Columbia University Medical Center in New York, N.Y. Of these, he said IABP is overwhelmingly the current standard of care, used in more than 70 percent of cases, but several recent studies (SHOCK II, BCIS I, CRISP AMI) have questioned the effectiveness of IABP in treating the largest patient subsets, the largest of which is cardiogenic shock, followed by high-risk PCI.
In addition to IABPs sold by Maquet and Teleflex, other percutaneous deployable technologies include Abiomed’s Impella family of catheter based pumps, CardiacAssist’s TandemHeart system and newer, portable and easier to use ECMO systems from Maquet and Thoratec. Jorde said none of these systems provide the ideal balance between ease of insertion, low profile, high flow and LV unloading, but the next generation of devices being developed may offer a solution.
Thoratec’s Percutaneous Heart Pump
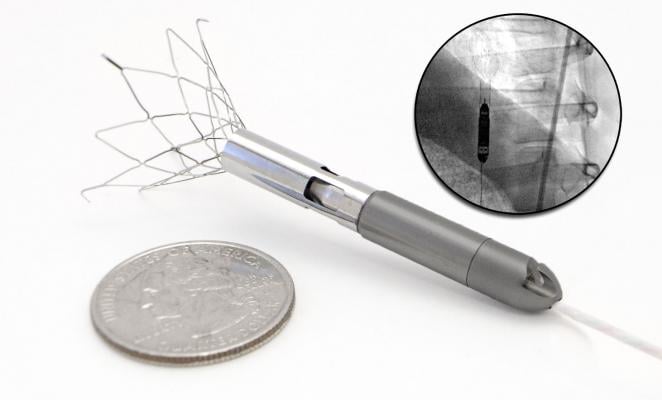
Jorde said Thoratec’s HeartMate PHP (percutaneous heart pump) might offer advantages over Impella and TandemHeart with an entry low profile, ease of use and a high rate of flow. It is delivered using a 13 French sheath and is unsheathed to expand to 24 French once delivered through the aortic valve into the left ventricle. The system uses a self-expanding stent-like covered cannula that houses a collapsible impeller. The cannula inlet is centered in the left ventricle, and the outlet is positioned in the aorta just past the aortic valve. He said it is designed to provide between 4-5 lpm of flow.
The device was tested first-in-man in February 2013. CE mark approval in Europe is expected by in 2015. Thoratec hopes to begin a pivotal U.S. trial for the HeartMate PHP in 2014 or 2015.
Reitan Catheter Pump
A transcatheter pump that deploys similar to an IABP in development is the Reitan Catheter Pump System. It is designed to treat acute decompensated heart failure patients, said its creator Oyvind Reitan, M.D. Ph.D., cardiology department, University Hospital Lund, Sweden. It uses a low-profile 10-French catheter for insertion using a 10 French femoral sheath and then extends a foldable propeller inside a protective cage. It is deployed in the descending aorta, in the same position as an IABP, and works in series with the heart, providing continuous flow. The portable driver system uses touch screen controls and a magnetic coupling system.
Reitan said the system is designed to address the fact that a majority of patients admitted with acute decompensated heart failure have significant renal impairment, which influences treatment and outcomes. In an initial 18-patient trial, the system was shown to increase perfusion pressure toward the kidneys and facilitated relief from congestion by removal of excess fluid in patients.
Right Heart Support
Right ventricular (RV) heart failure usually results in very poor prognosis, but pVADs may offer a quick and temporary solution to stabilize patients, said Jose Henriques, Ph.D., MBA, head of the cath laboratory at Academic Medical Center, University of Amsterdam, The Netherlands. He said part of the issue is when the RV enlarges, its changes the septal wall, causing it to push into the left ventricle (LV), decreasing LV function.
He said Abiomed and CardiacAssist both developed right ventricular assist systems.
The TandemHeart system used for LV support can be used in a different deployment configuration to RV support, with access via femoral/femoral veins or femoral/jugular veins. Its use in RV support was tested in a retrospective, observational registry of 46 patients receiving a TandemHeart for RV failure in eight tertiary care centers in the United States (Kapur N, et al. “Mechanical Circulatory Support for Right Ventricular Failure.” JACC Heart Failure, Vol. 1, Issue 2, April 2013). Data showed improved hemodynamic status from the registry of patients who have received this device, which may support the development of further prospective studies.
Abiomed’s Impella RP system is currently in clinical trials. It accesses the heart through the vena cava, through the RV and into the pulmonary artery. It is shaped differently than the left heart Impella devices to accommodate the right ventricular anatomy. Its impeller flow is also reversed from Impella LV devices to pump blood from the right atrium into the pulmonary artery toward the lungs. It includes a 22 French outflow.
Abiomed said the ongoing RECOVER RIGHT study for the Impella RP will be used to support a U.S. FDA humanitarian device exemption (HDE) submission. The company anticipates European CE mark approval in 2014, and FDA approval in 2015.
Henriques said both systems generate excellent flow, allowing for RV unloading and expects larger and multicenter experience will add to positive evidence.
New Treatments for Heart Failure
Start-up company Procyrion Inc. is developing a catheter-deployed pVAD intended for long-term use in the treatment of chronic heart failure (HF). The company said its 6 mm diameter Aortix device can be delivered via a catheter in a minimally invasive outpatient procedure lasting about 10 minutes.
Currently, long-term mechanical circulatory support (MCS) is limited to large, surgically implanted VADs, generally reserved for late stage heart failure. Procyrion hopes the Aortix will gain regulatory approval as a long-term circulatory support device in patients with earlier stage HF, helping head off worsening symptoms.
Aortix has been successfully tested with computer models, on the bench top in mock flow loops and in vivo in large animal HF studies. In three porcine experiments conducted last year, the pump was deployed in the thoracic aorta by standard cardiac catheterization techniques and was anchored with self-expanding struts. Pump support increased cardiac output by 10 percent, stroke volume by nearly 9 percent and ejection fraction by nearly 11 percent, while decreasing cardiac stroke work and afterload. Pump support enhanced renal perfusion through sustained increases in both renal artery flow (by 36 percent) and pressure (73 percent).
Abiomed has developed the Symphony device for heart failure patients, which it showed at TCT as a work in progress. It is an implantable counter pulsation device that is similar to an IABP, augmenting the heart’s own pumping action. It is designed for patients with Class III heart failure to help prevent them from progressing into Class IV.
Pediatric pVAD
At TCT 2013, Abiomed showed a work in progress Impella Pediatric, which is a miniaturized version of the adult Impella 2.5 device. This new version can better fit pediatric anatomy, especially the smaller aortic arch.



 August 14, 2023
August 14, 2023 









