
March 27, 2012 – Researchers found the antiplatelet drug abciximab significantly decreased damage to the heart muscle in patients with ST-segment-elevation myocardial infarction (STEMI), the most severe type of heart attack. Results of the INFUSE-AMI trial were presented at the American College of Cardiology’s 61st Annual Scientific Session and published simultaneously in the March 25 online issue of the Journal of the American Medical Association. Researchers also found that clot aspiration did not significantly reduce the damage to the heart muscle.
“Results from the INFUSE-AMI trial suggest that in patients with large heart attacks reaching the hospital early, the extent of heart damage can be reduced with a bolus of intracoronary abciximab delivered directly to the infarct lesion,” said lead investigator, Gregg W. Stone, M.D. He is professor of medicine at Columbia University College of Physicians and Surgeons, and director of research and education at the Center for Interventional Vascular Therapy at New York-Presbyterian Hospital. Stone also serves as co-director of the medical research and education division at the Cardiovascular Research Foundation.
The primary efficacy endpoint was heart attack size at 30 days, as measured by cardiac magnetic resonance imaging (MRI) for 382 of 439 surviving patients (87.1%). Abciximab was delivered locally to the site of the blocked artery using a novel catheter, the ClearWay Rx (Atrium Medical). Heart attack size was measured by calculating infarct size as a percentage of total heart muscle tissue. Patients in the two abciximab arms had significantly smaller heart attacks than patients in the two no-abciximab arms (median 15.1 vs. 17.9%).
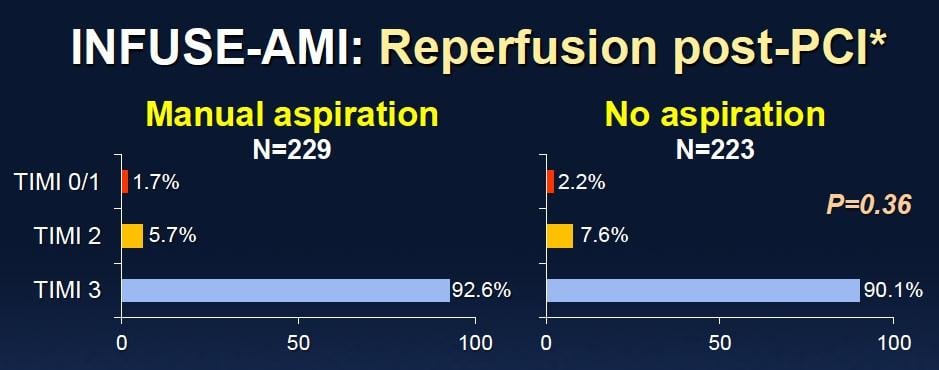
In contrast, aspiration thrombectomy did not significantly decrease heart attack size (17%) compared with no aspiration (17.3%). The aspiration plus abciximab group showed the lowest median heart attack size of 14.7%, compared with 17.6% for the other three groups combined. There were no significant differences among the four groups for any major measures of safety or efficacy at 30 days. Patients will be assessed again at six months and one year.
“A large randomized clinical trial of intracoronary abciximab delivered to the site of the infarct lesion through the ClearWay Rx catheter is needed to determine whether this therapy results in improved clinical outcomes without excessive bleeding,” Stone said.
“Additionally, in this trial, aspiration did not reduce infarct size. However, the final word about aspiration in STEMI will be spoken by the results from two large ongoing randomized trials of aspiration powered for mortality,” Stone added. These trials include TOTAL and TASTE.
The INFUSE-AMI trial used a 2 x 2 factorial design, isolating the impact of each intervention to maximize the chance of identifying any reduction in heart attack size. The trial, conducted at 37 sites in six countries, screened 6,318 patients and enrolled only 452 (7.2 percent) with large heart attacks presenting early, during the time when reduction in the size of the heart attack is still possible. The trial was a single-blind study in which the operator knew the randomization assignments.
All study subjects were treated with the anticoagulant bivalirudin, and randomly assigned to one of four arms: (1) aspiration plus abciximab, (2) aspiration without abciximab, (3) no aspiration plus abciximab, and (4) no aspiration or abciximab (control). In the two abciximab arms, a balloon catheter (ClearWay Rx) delivered the drug right to the site of the lesion, which invariably has thrombus present. Aspiration was accomplished through an Export (Medtronic) catheter. All patients who had angioplasty were also treated with bivalirudin, an anticoagulant that keeps bleeding and mortality low.
The INFUSE-AMI trial was funded by Atrium Medical, manufacturer of the ClearWay RX catheter, and Medtronic Inc., manufacturer of the Export catheter, and conducted by the Cardiovascular Research Foundation. Stone reported consulting fees from Abbott Vascular, Boston Scientific, Medtronic, Atrium, The Medicines Company, Merck, Daiichi Sankyo and Eli Lilly.
This study was simultaneously published in the Journal of the American Medical Association and released online at the time of presentation.
For more information: www.crf.org




 January 05, 2026
January 05, 2026 









