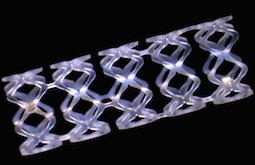
May 26, 2015 — Privately-held company Arterial Remodeling Technologies (ART) announced CE Mark clearance for its next-generation drug-free, pure bioresorbable scaffold used to treat coronary artery disease. The CE Mark was achieved following the completion of extensive pre-clinical research — this included up to three-years of follow-up, and supportive clinical results from leading coronary angioplasty centres such as the Hôpital Européen Georges Pompidou in Paris and investigators such as Jean Fajadet, M.D., at the Clinique Pasteur in Toulouse.
ART’s advanced bioresorbable scaffold is designed to provide a transient effective scaffolding that dismantles and relinquishes its primary mechanical function after three months, a commonly recognized requisite length of time necessary to allow the healing process to stabilize the artery. In addition the scaffold is designed to allow complete resorption of the polymer within 24 months. ART’s drug-free, pure bioresorbable scaffold is particularly suitable for the treatment of larger lumen coronary artery lesions.
Through an agreement concluded between the companies in March 2014, Terumo acquired exclusive acquisition rights for the coronary drug-eluting bioresorbable scaffold technology. The pure bioresorbable CE Marked scaffold developed by ART will serve as the platform for the next generation of coronary drug eluting bioresorbable scaffolds to be developed by Terumo.
Other indications, including the peripheral vascular application of the pure and drug-eluting scaffolds, are being developed by Vascular Bioresorbable Technologies (“VBT”) through an exclusive license agreement to further develop ART’s platform technology.
For more information: www.art-stent.com


 November 14, 2025
November 14, 2025 









