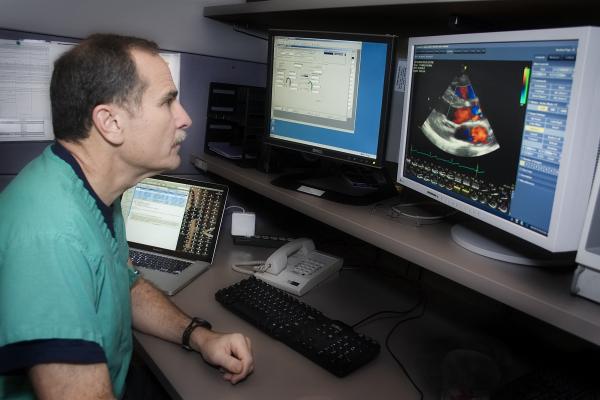
October 24, 2013 — Medical experts released “Guidelines for Performing a Comprehensive Transesophageal Echocardiographic Examination: Recommendations from the American Society of Echocardiography and the Society of
Cardiovascular Anesthesiologists.” This represents the first such update since the original transesophageal echocardiography (TEE) guidelines were published in 1999. Since that time, significant advancements in technology have expanded the use of TEE in clinical practice; this document reviews its updated clinical relevance in the operating room, intensive care unit, interventional lab and outpatient settings.
“The use of TEE has changed and the technology expanded so much over the past ten years, including the addition of three-dimensional TEE; it was imperative that we review and update our recommendations to reflect this extensive transformation in clinical practice,” said Rebecca Hahn, M.D, Fellow, American Society of Echocardiography (ASE), cardiologist, Columbia University and chair of the writing group for the new guideline.
Since the previous guideline’s publication, transesophageal probes and systems capable of producing real-time 3-D images have been developed and have achieved wide use. In addition, Hahn noted that “although the original guideline was widely adopted, it had limitations as it did not include some views that are needed to adequately examine some common cardiac disorders. Thus, the new document redefines a ‘Comprehensive TEE Examination’ and names an expanded set of 28 TEE views.”
The delineation of these views is intended to facilitate and provide consistency in training, reporting, archiving and quality assurance in the multiplane TEE examination. Other major changes in the new document include an extensive review of 3-D TEE protocols and a guide for TEE performed to address specific diagnostic questions.
Due to the proximity of the esophagus to the heart and cardiac vessels, TEE gives clinicians an excellent view into cardiac functioning, thus TEE has become an essential imaging tool for a variety of specialists including cardiac surgeons, anesthesiologists, cardiac interventionists and clinical cardiologists. It provides accurate information for a large range of diagnoses, especially for many catheter-based cardiac interventions.
In conjunction with the guideline document, ASE also released a poster based on the guideline recommendations. The poster acts as a quick reference guide in everyday practice.
For more information: www.onlinejase.com, www.asecho.org