August 20, 2012 — The Baylor Heart Hospital in Plano, Texas, recently became the first hospital in the world to merge two highly advanced technologies for atrial fibrillation (AF) therapy.
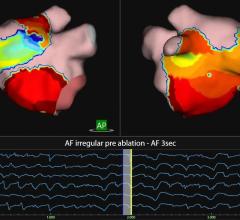
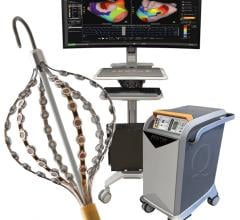
The procedure combined the use of multi-electrode mapping (MEM) software with the Epoch platform, making the full-service cardiovascular hospital the first in the world to do so. Introduced by Biosense Webster, the software allows the physician maneuvering a MEM-enabled catheter to acquire multiple mapping points simultaneously with a higher level of detail. The Epoch platform is an advanced computer-controlled technology that allows physicians to navigate within the patient's heart with robotic precision.
Merging these two technologies means higher levels of efficiencies in mapping and correcting the patient’s cardiac arrhythmias. J. Brian DeVille, M.D., FACC, FHRS, medical director of electrophysiology and an electrophysiologist on the medical staff of the Baylor Heart Hospital, said this first-in-the-world procedure is an important step to further advancing patient care in the arena of cardiac electrophysiology.
“The integration of these two technologies confirms the Heart Hospital Baylor Plano’s commitment to excellence in patient care and providing advanced treatment to our patients,” DeVille said. “Our goal is always to seek ways to further minimize risk to the patient, which then increases the probability of favorable outcomes.”
Mark Valentine, the hospital’s president, added, “The Heart Hospital is fully committed to improving patient outcomes and that involves implementing advanced technologies that will help us fulfill that goal. We consistently strive to be on the forefront when it comes to innovation because we know that ultimately that is what will serve our patients’ interests in positive ways and help us continue to provide safe, quality, compassionate care.”
For more information: www.thehearthospitalbaylor.com


 September 05, 2025
September 05, 2025