March 27, 2012 — Biodegradable polymer drug-eluting stents (DES) provide better long-term safety and efficacy than durable polymer DES, according to findings from an analysis of three major clinical trials — ISAR-TEST 3, ISAR-TEST 4 and LEADERS. The data were presented at at the American College of Cardiology’s 61st Annual Scientific Session.
The findings provide the first combined long-term data on the comparison between biodegradable polymer DES and durable polymer DES. Designed to improve long-term clinical outcomes while also shortening healing time, biodegradable polymer DES are a new generation of DES that have undergone little research and thus have yet to substantiate its claims. The three analyzed studies showed that after four years, use of biodegradable polymer DES resulted in lower rates of target lesion revascularization, definite stent thrombosis and cardiac death and heart attack than durable polymer DES.
“Because it is often difficult to design individual trials to test for differences in rarely occurring adverse events [like stent clotting], we pooled the data from the three largest trials to see if any differences between the two stent types could be seen,” said co-lead investigator Robert A. Byrne, M.B., B.Ch., Ph.D., a cardiologist at Deutsches Herzzentrum in Munich, Germany. “In addition, by including surveillance out to four years, this helped us better capture the differences between the two stents, as benefit was expected to first emerge with long-term follow-up.”
Among all three analyzed trials, 2,358 patients were randomly assigned to angioplasty with a biodegradable polymer DES (sirolimus-eluting = 1,501; biolimus-eluting = 857), while 1,704 patients were treated with a durable polymer SES (all sirolimus-eluting).
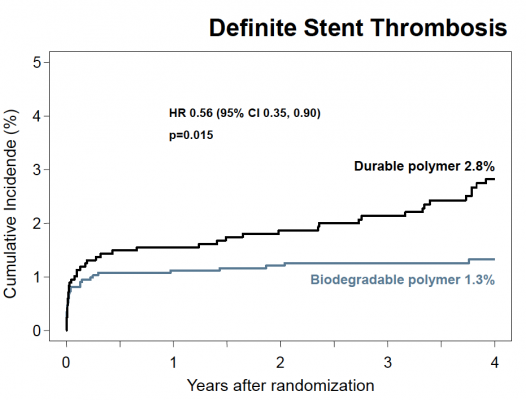
At the four-year follow-up point, the researchers found that the risk of target lesion revascularization (the study’s primary efficacy endpoint) was significantly lower among those patients treated with a biodegradable polymer DES than for those treated with a durable polymer DES (hazard ratio [HR] 0.82, 95 percent confidence interval [CI] 0.68-0.98, P=0.029). In addition, the risk of having a blood clot, called stent thrombosis (the study’s primary safety endpoint), was also significantly lower for those patients treated with a biodegradable polymer DES compared to those treated with a durable polymer DES (HR 0.56, 95 percent CI 0.35-0.90, P=0.015). This was driven by a lower risk of very late stent thrombosis (clots occurring more than one year after angioplasty) for the biodegradable polymer group (HR 0.22, 95 percent CI 0.08-0.61, P=0.004).
Furthermore, the incidence of heart attack late after stenting was lower for patients treated with biodegradable polymer versus durable polymer stents (HR 0.59, 95 percent CI 0.73-0.95, P=0.031).
While the arrival of DES has allowed interventionalists to provide treatment for more complex patients, concerns have arisen about the stents’ long-term safety, particularly concerning stent thrombosis. As a result, the polymer coating on the first-generation stents was targeted as an area for improvement. Specifically, the durable polymer remains in the coronary artery wall beyond the time when its useful function is served. This may cause delayed healing and a hypersensitivity reaction, leading to inflammation and stent thrombosis.
As a potential solution to these problems, new-generation stents with a bioabsorbable polymer were created. This polymer, which fully degrades and leaves a bare-metal stent in place, has been suggested to shorten healing time and cause less inflammation and subsequent stent thrombosis.
“These findings show that biodegradable polymer DES can provide better long-term safety and efficacy,” said Byrne. “This advantage, coupled with a shortened healing time compared with durable polymer DES, means that biodegradable polymer stents look to become an important tool for the interventional cardiologist in everyday practice.”
The current analysis was industry independent, supported in part by a grant from the Swiss National Science Foundation, and conducted at the ISAR Research Center in Munich, Germany, and the Clinical Trials Unit in Bern, Switzerland.
This study was simultaneously published in the European Heart Journal and was released online at the time of presentation.
The results offer a promising outlook for Boston Scientific's Synergy DES, now in development. It uses the same platform stent as the Ion and Promus, but instead of a duable polymer it uses abluminal biodegradable polymer containing everolimus. The company presented its first-in-man study at TCT 2011 and hopes to begin its EVOLVE II U.S. Food and Drug Administration (FDA) investigational decive exemption trial later this year.
For more information: www.acc.org


 January 05, 2026
January 05, 2026 









