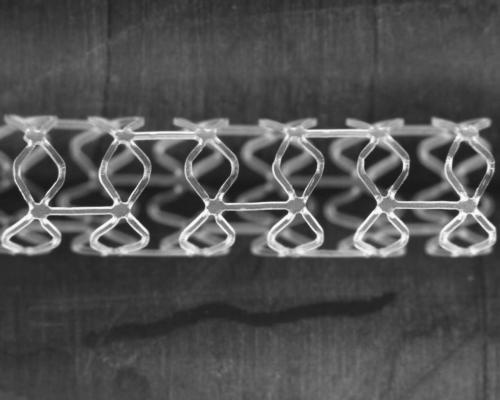
July 20, 2015 - Boston Scientific has initiated a study of the company's first fully resorbable drug-eluting scaffold system. The Fully Absorbable Scaffold Feasibility Study (FAST) is a prospective, single-arm study designed to assess the safety and performance of this next-generation scaffold for the treatment of atherosclerotic coronary lesions.
The first patients in the study were enrolled by Prof. Ian Meredith, primary investigator and director of MonashHeart, at Monash Medical Centre in Melbourne, Australia. The study will enroll up to 30 patients.
"By virtue of its unique design properties, incorporating thinner struts and enabling greater stent expansion while maintaining radial strength, this fully resorbable scaffold technology may potentially overcome a number of limitations with first-generation absorbable scaffolds," said Meredith.
The resorbable polymer scaffold incorporates several key design elements from the Boston Scientific Synergy stent system, including a resorbable polymer and an ultrathin everolimus coating which is applied abluminally (on the exterior) to the scaffold. It also features a delivery system built with Boston Scientific expertise designed to facilitate improved acute performance.
The Boston Scientific fully resorbable scaffold technology is under development and is not available for sale.
For more information: www.bostonscientific.com


 November 14, 2025
November 14, 2025 









