June 10, 2013 – Just a few years ago, integrated positron emission tomography and magnetic resonance (PET/MR) imaging was found only in research institutes, but little by little the technology has expanded into clinical practice. This is especially true for cardiac indications, for which the highly sensitive soft tissue contrast of MR and the functional and metabolic imaging of PET are particularly valuable. New research proves the value of PET/MR compared to PET/computed tomography (CT) in cardiac applications, say researchers at the Society of Nuclear Medicine and Molecular Imaging’s 2013 Annual Meeting.
PET/MR imaging techniques have advanced during the past several years, particularly in terms of disease detection and the correction of blurring (attenuation). PET/CT systems use CT to compensate for attenuation, and PET/MR employs MR. Part of this study compared the two to see how they match up. “Our research demonstrated that cardiac PET assessment for heart muscle viability using PET/MR yielded comparable results to PET acquired using PET/CT,” said principal author Jeffrey M.C. Lau, MD, PhD, from Washington University in St. Louis, Mo. “It showed that MR can be used for PET attenuation correction in the same way that CT can.”
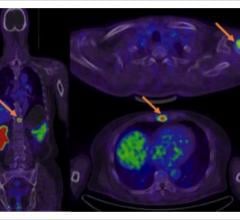
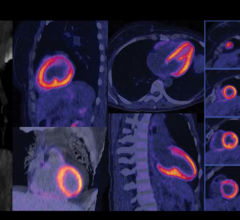
This throws open doors for the technology in a variety of cardiac applications. In this instance, researchers used an imaging agent called F-18 fluorodeoxyglucose (F-18 FDG), which mirrors glucose as a source of fuel for heart and other cells that metabolize the agent readily. F-18 FDG-PET scans tell cardiologists about the metabolic activity of cardiac tissues and overall heart muscle function. This study focused on visualization of FDG in cardiac cells by PET/MR compared to PET/CT, which was found to be very similar. Comparable attenuation correction and imaging agent uptake measurements move PET/MR forward toward important clinical applications, including the use of PET/MR for imaging scarring of heart tissue and subsequent complications after cardiac arrest, with some major benefits for patients.
“Our research provides the groundwork for future research in cardiac PET/MR imaging,” said Lau. “PET/MR provides powerful cardiac imaging and requires a lower radiation dose than PET/CT. Also, the MRI component, which can be acquired simultaneously, provides excellent heart muscle signal for imaging scar tissue caused by heart attacks. In particular, our group is most interested in applying the PET/MR technology to evaluate the likelihood of arrhythmia or irregular heart beat development in patients who have had heart attacks.”
The study included 31 patients with no history of heart problems being screened for cancer. All subjects underwent both PET/CT and PET/MR with F-18 FDG injection administered about an hour before PET/CT and two hours before PET/MR. Attenuation correction with MR was made possible by a specialized dual-echo MR sequence that orders radiofrequency magnetic fields interacting with atomic nuclei in the body to “see” differentiation between water and fat. The uptake of FDG in the myocardium, or heart muscle wall, was measured by looking at a cross-section of the left ventricle of the heart that empties oxygen-rich blood into the aorta. The average measurement of FDG uptake in the left ventricle was nearly identical, 4.68 for PET/MR and 4.62 for PET/CT. This research also has implications for future studies into ischemic cardiomyopathy, which is a weakening of the left ventricle that can lead to reduced blood flow from the heart and possibly life-threatening cardiac events.
Studies have shown that implanted cardiac defibrillators (ICD) are related to the development of ventricular arrhythmias in up to a third of implanted patients within three years. Researchers hope to glean more information about this relationship with future PET/MR studies.
“Our hope is that in the future PET/MR will become the imaging modality of choice for certain cardiac diseases,” says Lau. “One potential use of cardiac PET/MR is to guide the patient selection process when deciding if patients who have suffered ischemic cardiomyopathy are good candidates for cardiac defibrillator implantation. We hope that a better understanding of the metabolic and anatomic correlation of PET/MR in the myocardial scar and scar border can provide more insight into arrhythmias that lead to sudden cardiac death.”
Further studies are needed to continue providing information about the benefits and appropriateness of PET/MR in clinical practice. This study was conducted in conjunction with Siemens Medical Solutions.
Scientific Paper 27: Jeffrey Lau, Shivak Sharma and Luciano Amado, Department of Cardiology, Washington University in St. Louis, St. Louis, MO; Richard Laforest, Jonathan McConathy, Robert Gropler and Pamela Woodard, Department of Radiology, Washington University in St. Louis, St. Louis, MO; and Agus Priatna, Siemens Medical Solutions, Malvern, PA, “Feasibility of MRI attenuation correction in cardiac-gated FDG-PET,” SNMMI’s 60th Annual Meeting, June 8–12, 2013, Vancouver, British Columbia.
For more information: www.snmmi.org

 June 23, 2025
June 23, 2025