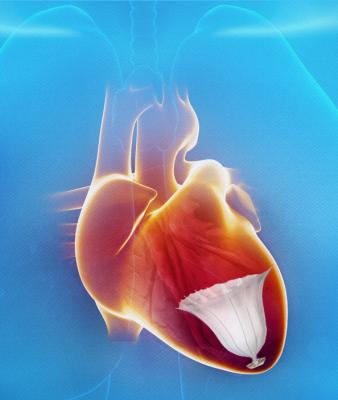
CardioKinetix Initiates Landmark Study of First-of-Its-Kind Treatment for Heart Failure in the United States
January 18, 2013 — CardioKinetix Inc. announced the enrollment of the first patients in PARACHUTE IV, the randomized pivotal U.S. clinical trial of the minimally invasive Parachute Ventricular Partitioning Device for the treatment of heart failure.
PARACHUTE IV is a multi-center pivotal trial designed to evaluate the PARACHUTE implant vs. optimal medical therapy (randomized 1:1) in approximately 500 patients with ischemic heart failure at up to 65 centers. The event-driven primary endpoint includes all-cause mortality and hospitalization for worsening heart failure. Other key endpoints include functional outcomes, quality of life and hemodynamic measures by echocardiography.
The first implant with the Parachute device was performed by David Mego, M.D., assistant medical director at Arkansas Heart Hospital in Little Rock, Ark., who treated a 39-year-old female with NYHA class III heart failure referred by Carl Leding, M.D., director of the Congestive Heart Failure Clinic at Arkansas Heart Hospital.
Marco Costa, M.D., Ph.D., director of the Interventional Cardiovascular Center and the Research and Innovation Center at the Harrington Heart and Vascular Institute at Case Western Reserve University and co-principal investigator of the trial, said, “I am thrilled to be pioneering this exciting therapy in the United States, where there is a significant need for better treatments for this heart failure patient population.”
“Many patients who have had a heart attack experience an extremely poor quality of life due to the debilitating symptoms of heart failure, including shortness of breath, fatigue, lack of appetite, impaired thinking and increased heart rate. A classic example is the patient just treated at Arkansas Heart Hospital, where the patient experienced a heart attack less than two years ago and now struggles with simple things like dressing, cooking or playing outside with her son because of shortness of breath and extreme fatigue,” said William T. Abraham, M.D., the co-principal investigator of the trial and director of the Division of Cardiovascular Medicine and professor of internal medicine, physiology and cell biology at The Ohio State University Medical Center. “In October, three-year data on the initial patients treated with Parachute were presented at the TCT Conference by Costa. These strong and encouraging results provide confidence to initiate a landmark randomized, pivotal trial where a positive outcome will establish this technology as a primary therapy option for the treatment of ischemic heart failure patients.”
The Parachute device offers the first minimally invasive catheter-based treatment to partition the coronary muscle damaged by a heart attack. The treatment excludes the non-functional heart segment from the healthy, functional segment to decrease the overall volume of the left ventricle and restore its geometry and function.
“This initiation of the U.S. randomized pivotal study is a major milestone for CardioKinetix,” said Maria Sainz, president and CEO of CardioKinetix. “We are excited about the opportunity before us to make a major positive impact for patients with heart failure, and we believe that we have partnered with the right heart failure physicians and interventional cardiologists to enroll this trial quickly.”
For more information: www.cardiokinetix.com.


 January 05, 2026
January 05, 2026 









