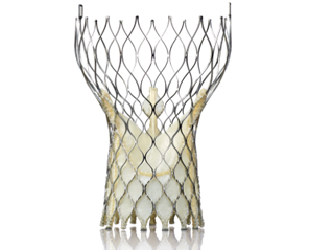
November 1, 2013 — In a clinical trial, the Medtronic Corevalve self-expanding
transcatheter aortic valve met the key performance objective of reducing death and stroke in patients with severe aortic stenosis at “extreme risk” for surgery. Results of the CoreValve Extreme Risk trial were presented at the 25th annual Transcatheter Cardiovascular Therapeutics scientific symposium (TCT 2013) in San Francisco.
The CoreValve Extreme Risk trial was a prospective, multicenter, controlled, non-randomized, single-arm investigation evaluating the safety and efficacy of the transfemorally implanted CoreValve self-expanding transcatheter
heart valve. The trial was conducted in 487 patients with symptomatic, severe aortic stenosis.
All patients were deemed to be at extreme risk for surgical aortic valve replacement, and were thus treated with the Corevalve device. The primary endpoint was a composite of all-cause mortality or major stroke rate at 12 months; the Kaplan-Meier determined event rate was compared with a pre-specified objective performance goal (OPG).
Of the 487 patients enrolled in the study, 471 had an attempted implantation and were designated as the primary "as treated" analysis population. Patients were elderly (83.1 years), and were severely symptomatic (NYHA class III or IV, 91.9 percent). The Society for Thoracic Surgery predicted risk of mortality was 10.3 percent ± 5.6 percent and was > 15 percent in 17.6 percent of patients.
At 12 months, the composite rate of death or major stroke was 25.5 percent, significantly below the 95 percent confidence interval of the performance goal, which was set at 43 percent. While moderate paravalvular leak was observed in 11 percent of patients at one month, 80 percent of patients with moderate paravalvular (PV) leak at one month who survived to one year experienced a reduction in PV leak over time.
“The CoreValve Extreme Risk study achieved its primary endpoint of a reduction in all cause mortality or major stroke at one year compared to a rigorously defined OPG,” said Jeffrey Popma, M.D., lead investigator, director, Interventional Cardiology, Beth Israel Deaconess Medical Center.
Medtronic has three FDA IDE trials evaluating the Corevalve for high-risk and extreme-risk surgical patients and in patients at intermediate risk for open-heart aortic valve replacement (SURTAVI Trial).
For more information: www.crf.org, www.medtronic.com