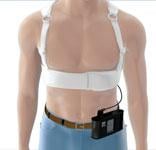
The LifeVest protects patients from sudden cardiac death following a heart attack, before or after bypass surgery or stent placement, and for those with cardiomyopathy or congestive heart failure.
Oklahoma Heart Hospital (OHH), Oklahoma’s first dedicated heart hospital, has excelled at creating quality outcomes through an integrated approach to patient care. To maintain this culture of quality, our team of more than 60 physicians, including interventional cardiologists, general cardiologists, cardiothoracic surgeons and electrophysiologists, practice a systematic process of patient care that includes high adherence to a set of core measures or indicators. Specifically, managing a patient’s risk for sudden cardiac death (SCD) is a critical piece of the care we provide.
Acute cardiac events, such as hospitalization for myocardial infarction or newly diagnosed heart failure, regardless of etiology, are associated with high mortality in the early months following a cardiac event, a large proportion of which are due to SCD. It has been shown that 10 percent of high-risk patients experience ventricular tachycardia (VT)/ventricular fibrillation (VF) within 30 days after a coronary artery bypass graft (CABG) procedure and 6 percent die or have ventricular arrhythmias within the first 90 days post-CABG.[1,2] Likewise, baseline left ventricular ejection fraction (LVEF) is the most powerful predictor of long-term mortality in percutaneous transluminal coronary angioplasty (PTCA) patients, with the most mortality occurring during the first three months in the high-risk group.[3] The presence of heart failure in post-myocardial infarction (MI) patients results in four to six times greater risk for SCD.[4]
Based on this high early risk, we believe it is critical that patients are screened for SCD risk early after a cardiac event in order to develop the treatment plan that best addresses the patient’s individual needs. Our understanding of the predictive value of a low LVEF following a cardiac event is central to improvements we have made in screening patients for SCD. At OHH, patients with an ejection fraction of ? 35 percent are screened for SCD risk and physicians of all specialties are empowered to lead in this effort.
Assessing Patient Need for an ICD
Managing a patient’s SCD risk requires consideration of numerous factors including dietary adjustment, medication titration and an assessment of implantable cardioverter defibrillator (ICD) implantation waiting period criteria (40 days post-MI and 90 days post-PTCA or CABG). We believe there is clinical value in giving the patient time to recover and respond to medical therapy. Our experience has shown that a portion of patients can experience meaningful LVEF improvement, and therefore not require long-term SCD risk protection. Our practice has taken active steps to manage the patient’s SCD risk during the implantable cardioverter defibrillator (ICD) implantation waiting periods that include conducting an internal audit to ensure we are in compliance with current waiting period criteria, utilizing our electronic medical record (EMR) system to ensure clear visibility of the patient’s treatment path to all caregivers, and providing interim SCD protection by prescribing a wearable cardioverter defibrillator (WCD).
Temporary Protection From a Wearable Defibrillator
At OHH, we utilize the WCD to address the patient’s need for SCD protection in the early period following their cardiac event. We utilize the WCD to ensure the patient is protected while monitoring for clinical improvement or waiting to receive permanent long-term SCD protection with an ICD. Our colleague, Mark Harvey, M.D., had the first patient to be saved in the United States by the WCD during the clinical trial that led to U.S. Food and Drug Administration (FDA) approval. To date, the WCD has saved the lives of 13 OHH patients. This significant achievement has been impactful to not only the patients saved but also to our physicians. The experience of prescribing a WCD and having it save the life of a patient is a powerful reminder of the very real risk of SCD that certain patients face when they leave the safety of the hospital and return home.
Our current thinking regarding the role of the WCD at OHH is an example of how our collaborative approach to patient care evolved as the use of and belief in the technology increased over time. Years ago, when our experience with the WCD was still limited, the electrophysiologist consulted on every potential patient. Over time, our use of the WCD has increased along with the importance we give to screening patients with low ejection fractions in the early risk period. Today, general cardiologists, heart failure specialists, thoracic surgeons and interventional cardiologists are screening for SCD and prescribing the WCD directly. From a patient flow perspective, it simply makes sense. We have demonstrated that early action by an empowered and educated physician better ensures that more patients are assessed for SCD risk, which in turn reduces the chance of an at-risk patient being discharged unprotected.
The symbiotic relationship between the WCD and ICD in complete management of our patients’ SCD risk is significant in the broader continuum of cardiac care for patients with low ejection fractions. The utilization of both technologies ensures comprehensive SCD protection in the near- and long-term, and information captured by the WCD helps us make better informed decisions about a patient’s care. As an added benefit, the WCD serves as a reminder to many patients of the seriousness of their condition and that follow-up care is needed. In the old paradigm, patients went home and, while we made every effort to ensure they were not lost to follow-up, we had to wait until they returned to be reassessed. We had no optic into whether their clinical status was improving or worsening. Now, the WCD gives us the ability to access data (e.g. compliance information, electrocardiogram (ECG) capture, etc.) that is used to impact downstream clinical decisions while serving as a reminder to the patient to return for assessment. For example, the ability to potentially capture the presence of an atrial or ventricular arrhythmia can affect the type or timing of ICD implantation, respectively.
We believe that the management of SCD risk following a cardiac event is a critical component of a patient’s overall cardiac care. Improving care and outcomes, as we know, cannot occur in a vacuum. We are proud of the model we have constructed at OHH and believe that the combination of improved screening procedures, advances in technology, and a collaborative approach to patient care has elevated the level of SCD risk management and ultimately saved lives.
Editor’s note: Jack L. Collier, M.D., has been with Oklahoma Heart Hospital since 2008, specializing in cardiac electrophysiology and pacing. He attended University of Oklahoma College of Medicine and has previously practiced with University of Oklahoma, St. Louis University Medical Center and University of Arkansas for Medical Services.
Derek Norman, M.D., has been with Oklahoma Heart Hospital since 2008, specializing in interventional cardiology. He attended University of Oklahoma College of Medicine and has previously practiced with University of Oklahoma and University of California.
References:
1. Toda K et al. “Revascularization in Severe Ventricular Dysfunction (15%?LVEF?30%): A Comparison of Bypass Grafting and Percutaneous Intervention.” Annals of Thoracic Surgery 2002;74: 2082-2087.
2. Kaul TK et al. “Ventricular Arrhythmia Following Successful Myocardial Revascularization: Incidence, Predictors and Prevention.” Eur J Cardiothorac Surg 1998;13: 629-636.
3. Halkin A et al. “Prediction of Mortality After Primary Percutaneous Coronary Intervention for Acute Myocardial Infarction: CADILLAC Risk Score.” JACC 2005;45: 1397-1405.
4. Solomon SD et al. “Sudden Death in Patients with Myocardial Infarction and Left Ventricular Dysfunction, Heart Failure, or Both.” NEJM 2005;352: 2581-2588.



 January 13, 2026
January 13, 2026 









