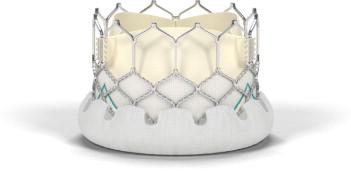
January 28, 2014 — Edwards Lifesciences Corp. received CE marking for its Edwards Sapien 3 transcatheter aortic valve.
The Sapien 3 valve has an outer skirt providing a seal to address paravalvular leak and can be delivered through a low-profile 14 French expandable sheath (eSheath), which has shown through early clinical experience a low rate of complications. The valve can be implanted through multiple approaches: transfemoral, transapical or transaortic. Once implanted, the discreet valve anchors in the aortic annulus.
In the United States, the Edwards Sapien 3 valve is an investigational device being studied in the PARTNER II
Trial and is not yet available for sale in the country.
For more information: www.edwards.com