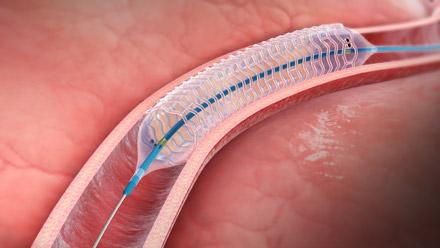
May 16, 2013 — Elixir Medical Corp. announced it received CE (Conformité Européenne) mark approval for its DESolve Novolimus-eluting bioresorbable coronary stent scaffold system. The scaffold is designed to degrade in about one year returning the patients’ coronary vessel ultimately to its normal de novo state.
“The CE mark approval for DESolve brings to the market a differentiated product platform with important advantages,” said Martin Leon, M.D., New York – Presbyterian Hospital/Columbia University Medical Center, New York, N.Y., and chairman of Elixir Medical’s DESolve Scaffold Program. “Elixir’s DESolve may help to transform the interventional treatment of patients with coronary artery disease by providing optimal vessel support when needed and degrading in about a year leaving the vessel free of a permanent metallic implant.”
Results of the international pivotal DESolve Nx trial, designed to enroll 120 patients at 15 centers in Europe, Brazil and New Zealand were submitted as part of the CE mark application, and will be presented in Paris in the Main Arena of the Palais Des Congres de Paris at the “From Late Breaking Trial to Clinical Practice” session of EuroPCR 2013 Tuesday, May 21. The DESolve Nx trial represents one of the largest bioresorbable scaffold clinical trials with QCA (quantitative coronary angiography) follow up in the industry to date.
The primary safety endpoint of the DESolve Nx trial is the composite of major adverse cardiac events (MACE) comprised of cardiac death, target vessel myocardial infarction (MI) and clinically indicated target vessel revascularization (TLR). The primary angiographic endpoint of the trial is in-scaffold late lumen loss at 6 months as assessed by QCA (quantitative coronary angiography). In a sub-set of patients, additional QCA assessment will be conducted at 24 months; scaffold and vessel assessment using intravascular ultrasound (IVUS), optical coherent tomography (OCT) will be also conducted at baseline, six and 24 months; computed tomography will also be conducted at 12 months, providing long-term assessment of the scaffold.
“The CE mark for DESolve is a major milestone that confirms the impressive results of the device performance observed in the clinical setting,” said Stefan Verheye, M.D., Ph.D., ZNA Middleheim Hospital, Antwerp, Belgium, and co-principal investigator of the DESolve Nx study. “Having used the DESolve bioresorbable scaffold system in the first-in-man study and observed its outstanding performance in the clinic during the subsequent DESolve Nx pivotal trial, I am confident that Elixir’s fully bioresorbable DESolve system is poised to lead the next frontier of interventional cardiology innovation.”
“The CE mark approval of Elixir’s DESolve represents a major achievement in interventional cardiology as it brings together important performance characteristics that physicians are seeking for the better treatment of their patients,” said Alexandre Abizaid, M.D., Ph.D., Instituto Dante Pazzanese de Cardiologia in Sao Paulo, Brazil, and co-principal investigator of the DESolve Nx trial. “DESolve’s unique characteristics include its ability to maintain radial strength and vessel support for the critical period of vessel healing, the ability to self-appose to the nominal vessel wall, its excellent scaffold expansion range, and its ability to degrade in the body in about a year.”
The approval fulfills a goal by the maker to offer a comprehensive product portfolio of three CE mark approved drug-eluting systems spanning all coronary stent market segments – including a novolimus-eluting metal stents with both biodegradable polymer and durable polymer, and now a bioresorbable stent.
Elixir intends to initiate commercial sales of DESolve in a broad range of sizes in select international markets later this year.
The DESolve scaffold, developed from a proprietary and proven poly-L lactide (PLLA)-based polymer, provides optimal strength and support to the artery while delivering the novel anti-proliferative drug, novolimus. The unique attributes of the DESolve scaffold system include (a) its ability to self-appose to the nominal vessel wall size in cases of malapposition; (b) its ability to maintain radial strength and vessel support for the necessary period of vessel healing while degrading in about a year; and (c) its ability to have a wide margin of expansion.
For more information www.elixirmedical.com


 November 14, 2025
November 14, 2025 









