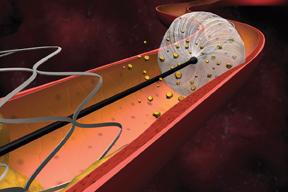
Invatecs FiberNet Embolic Protection System (EPS) is indicated for the treatment of patients receiving endovascular intervention for carotid artery disease.
When stenting emerged as a minimally- invasive treatment for clogged arteries associated with peripheral artery disease (PAD), medical experts raised concerns that delivery of stents through the vasculature could potentially knock loose plaque built up on the inside of the artery walls. This loose plaque can become emboli that travel through the bloodstream and cause severe complications, most notably stroke. This concern is particularly salient in carotid artery stenting, which led to the development of embolic protection devices, designed to prevent emboli traveling to the brain and causing stroke.
Embolic protection devices act as a trap for emboli, preventing them from traveling through the bloodstream. Three types of embolic protection devices are available to physicians: filter baskets, distal occlusion balloons and proximal occlusion balloons.
Of the three, filter baskets are used in the vast majority of peripheral artery stenting procedures. Filter baskets are net-like structures that are delivered past the lesion site in order to catch emboli that may be dislodged during stenting or angioplasty. Its mesh composition allows blood to continue to flow through the artery during the procedure, thereby reducing patient discomfort. Despite this advantage, several questions have been raised in regard to filter baskets. For example, because of the net-like structure, it is suspected that smaller emboli may be able to pass through the holes and continue traveling through the bloodstream. In addition, if the filter basket is not properly sized to the diameter of the vessel, apposition may be insufficient and emboli may be able to travel around the rim of the basket. A third potential limitation of the filter basket is the possibility that emboli trapped within the basket may clump together and consequently block blood flow. Finally, these devices have a larger profile than other types of embolic protection devices. Because they have to be delivered past the site of plaque buildup, there is a risk that the device itself will knock emboli loose. Despite these concerns, filter baskets have shown extremely high capture efficiencies in clinical trials, and the rates of major adverse events have been low.
Like filter baskets, distal occlusion balloons are also delivered past the lesion site, but rather than trap the emboli, distal occlusion balloons are inflated within the lumen, completely blocking off the artery and preventing emboli from traveling in the bloodstream. After stenting, the emboli are aspirated out of the occluded portion of the vessel, the balloon is deflated, and blood flow returns to normal. Distal occlusion balloons address some of the questions raised regarding filter baskets: because the balloons have a smaller profile than filter baskets, there is less concern that they will knock plaque loose when they are delivered past the lesion. As well, balloons can be more easily fitted to the size of the vessel, therefore providing a more complete blockage. Because blood flow is completely blocked off, however, patients have reported discomfort when treated with these devices, hence there is a greater popularity of filter baskets.
The third type of embolic protection device, the proximal occlusion balloon, attempts to address the concerns associated with the previous two devices. Proximal occlusion balloons are placed before the lesion site to block off blood flow, thereby preventing plaque from traveling upstream into the brain during a carotid stenting procedure. The mechanism of action of proximal occlusion balloons is to reverse blood flow in the internal carotid artery (which supplies blood to the brain) by blocking off the common carotid artery, which creates a negative pressure gradient. In order to prevent redirection to the external carotid artery (which supplies the face with blood), a second balloon is used to occlude this branch. Because the balloons are delivered ahead of the lesion site, there is no risk that device delivery itself will knock emboli loose. The primary limitations associated with proximal occlusion balloons are the difficulty of the procedural technique and the lack of clinical evidence supporting the advantage of proximal occlusion balloons over filter baskets. Although emboli, in theory, can pass through the holes of a filter basket, clinical trials have failed to illustrate that these small particles are capable of causing stroke.
Many clinical trials have evaluated the efficacy of embolic protection devices for their ability to prevent stroke during carotid stenting procedures given that manufacturers often develop, test, and package embolic protection devices and carotid stents together. In the ACT 1 clinical trial, for instance, Abbott Vascular is examining the noninferiority of stenting with embolic protection – achieved with the company’s Xact carotid stent and Emboshield filter basket – to surgical treatment of carotid artery disease. Similarly, Boston Scientific is comparing the effectiveness of surgical treatment to carotid stenting with its WALLSTENT and FilterWire embolic protection device. In the SAPPHIRE trial, Johnson & Johnson subsidiary Cordis is evaluating the effectiveness of its ANGIOGUARD XP distal protection device in preventing stroke during carotid stenting procedures employing the company’s PRECISE carotid stent. Invatec continues to evaluate its Mo.Ma proximal occlusion balloon through the ARMOUR clinical study. The results of these trials, and many others, support not only the use of embolic protection, but also the minimally invasive treatments that accompany them.
Although the industry has focused mainly on the use of embolic protection devices in the prevention of stroke, the devices are also used during coronary and renal stenting procedures, albeit in much lower volumes. In the coronary indication, embolic protection devices are restricted to percutaneous interventions on saphenous vein grafts. When a patient undergoes a surgical bypass procedure using a vein graft, the graft may subsequently become occluded because its anatomy is different than that of normal coronary arteries, making it more susceptible to emboli formation. For that reason, when a coronary stenting procedure is performed inside a saphenous vein graft, embolic protection is often used. However, a major complication prevents more widespread use in CABGs – the embolic protection device can catch on the deployed stent during removal, negatively affecting the placement and therefore the effectiveness of the stent.
Embolic protection often accompanies renal stenting in order to prevent emboli from traveling into the kidneys. Renal stenting remains controversial, however, as some clinical studies have actually shown a decrease in kidney functioning following a stenting procedure. Physician interest in the procedure has subsequently waned, and therefore renal procedures account for a small portion of embolic protection device usage. Nonetheless, companies like Johnson & Johnson and Centocor – which are cosponsoring a clinical trial examining the effectiveness of renal artery stenting, both with and without distal embolic protection – continue to examine the efficacy of stenting in an effort to garner support for the minimally-invasive treatment of renal artery stenosis.
Recent trends are pointing to a growing interest in the use of embolic protection devices in new indications and procedure types, most notably with lower-extremity stenting and atherectomy procedures. Some experts suggest that emboli in the lower extremities can travel through narrower and narrower arteries until eventually the emboli become trapped and consequently occlude blood flow to a part of the body. As a result, a number of lower-extremity stent manufacturers are expanding R&D efforts to include embolic protection devices.
Atherectomy is becoming a more popular treatment for PAD. This technique cuts built-up plaque away from the artery wall, and although the particles of plaque are aspirated, the possibility exists for smaller particles to evade capture and travel down the bloodstream. In 2008, medical device manufacturer ev3 began a clinical trial, DEFINITIVE Ca++, examining the efficacy of the SilverHawk atherectomy device with its SpiderFX embolic protection device in the treatment of lower-extremity arterial disease. The results of this study will significantly impact the use of lower-extremity atherectomy, and consequently the use of embolic protection in this indication. Given the growing popularity of atherectomy, companies that can show positive results with embolic protection devices employed in these procedures stand to benefit.
While embolic protection devices serve an important function to reduce procedure-related complications, the adoption and use of these devices will continue to be affected by the trends that influence the uptake of stenting. As minimally-invasive treatment options become more commonplace, the use of embolic protection devices – perhaps in novel indications – will continue to expand.
Editor’s note: Millennium Research Group (www.MRG.net), a Decision Resources Inc. company (www.DecisionResourcesInc.com), is the global authority on medical technology market intelligence and the leading provider of strategic information to the healthcare sector. The company provides specialized industry expertise through syndicated reports, ongoing Marketrack projects, customer loyalty tracking, facility-level procedure forecasting, and customized solutions.




 April 25, 2023
April 25, 2023 









