Cappella's Sideguard ostium-protection device for bifurcation stenting.
Are drug-eluting stents destined to fail?
In Part 1 of this investigation, the connection of DES and thrombogenicity was raised, and this perplexing drawback was explored, in part, through the views of 2005 ACC presenter Renue Virmani, M.D., FACC, medical director at CVPath. She asserts that the culprit in DES-related thrombosis is likely the polymer rather than the drug contained in the DES, which then begs the question: Can the amount of drug and polymer applied to stents be reduced?
Innovative approaches may be called for.
According to Donald S. Baim, M.D., interventional cardiologist at Boston's Brigham and Women's Hospital and a professor of Medicine at Harvard Medical School, reducing drug-polymer loading on DES would be beneficial. He believes that using bioerodable rather than biostable polymers and applying the drug to the ablumenal (or vessel wall side) stent surface might provide the same therapeutic benefit as coating the entire stent. Dr. Baim uses Conor Medsystems and Labcoat as examples.
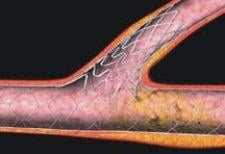
Significant decreases in stent thrombosis may lie in third-generation stents, such as Conor Medsystems' Costar stent. The Costar is a new alloy bare-metal stent with reservoirs (or wells) bored directly into the stent struts. The wells can be loaded with drugs and polymers, allowing for directional drug delivery (or ablumenal) or bidirectional drug delivery into both the vessel wall and the lumen. With the Conor stent, no polymer (bioerodable) is left behind. This could potentially reduce stent thrombogenecity, and in theory, should not interfere with the vessel's healing process because all that would remain is a bare metal stent once the drug polymer is released.
Unlike DES, bare-metal stents are rapidly covered by endothelial cells because there is no drug or barrier to interfere with the vessels' healing process, says Dr. Baim.
However, a potential limitation exists with the Conor stent design: The stent struts are thick. In order to make room for the reservoirs and to maintain radial strength, Conor had to develop a stent with thicker struts. Thicker stent struts have had a tendency to cause more vessel wall damage because, following deployment, the struts occupy a larger surface area. Vessel wall damage can lead to platelet activations and aggregation, which can cause thrombi to form. Nevertheless, Dr. Baim says that “Conor [has] seen good clinical results with their well design released ablumenally.”
New Solutions
Labcoat has developed a method for coating practically any commercially available coronary stent on the market. The company uses jet technology to precisely apply microstructures of drugs and a proprietary bioerodable polymer to the ablumenal surface of stents.
“Labcoat's Juxtaposed Ablumenal Coating Process (JAC Process) is capable of exclusively coating the ablumenal side of pre-mounted bare metal stents using a minimal amount of drug and polymer and leaving all other stent surfaces free of coating,” states the company's Web site. The advantage of applying the drug and polymer ablumenally using the JAC Process reduces the consequences of excessive and unwanted polymer, including cracking and/or webbing.
Dr. Baim says that Labcoat's jet technology can apply as little as 30-40 mcg of polymer, which is a significant decrease compared to first-generation DES that have 1,000 mcg or more of polymer that remains on the stent once the drug is eluted.
“This raises the possibility of promoting endothelial coverage,” Dr. Baim continued, referring to a Labcoat-coated stent. He believes that Labcoat-coated stents could be the solution to the stent thrombosis issue.
Other companies that are looking toward polymer-free or polymer-light stents as an approach to next-generation stenting are Biotronik, Orbus-Neich, and Biosensors, to name a few. Their stents will minimize the amount of drug and polymer applied to stent surfaces. As Dr. Baim indicates, such an approach might provide a solution for reducing stent thrombosis. Some of the newer technologies, even prototypes, are already capturing the attention of some of the major stent players in the market.
Biotronik has been coating its stents with silicon carbide. Silicon carbide coating reduces fibrinogen activation when fibrinogen comes into contact with a metallic surface, such as the metallic surface of a stent. Silicon carbide has been used to coat other medical devices, such as orthopedic joints, to minimize device-induced thrombi formation.
Other surface coating modifications, such as endothelial progenitor cells (EPC), may play a significant role in providing a viable therapeutic alternative to polymers that may not be tolerable in some patients.
Orbus-Neich is one of several companies that is in line to obtain CE Mark (European approval) for its stenting technology. The EPC Genous stent's bioengineered surface utilizes the body's antibodies to capture the patient's own circulating endothelial progenitor cells. Once captured, the EPCs rapidly form an encapsulating endothelial layer over the stent, which helps to inhibit restenosis and reduces the likelihood of stent thrombosis.
MIV Therapeutics is another company offering an alternative to polymer. It is using hydroxyaptite (HAp) films and composites in the development of biocompatible coatings for stents and drug delivery systems.
Biosensors International is working on two stent technologies:
the BioMatrix DES and the AXXION DES. Biosensors' BioMatrix DES will feature a nonpermanent polymer coating that will co-release its drug and polymer, returning the stent to bare metal once its payload is released. The BioMatrix will be a polymer-light stent as the polymer is only applied ablumenally.
The AXXION has a CALIX biocompatible, passive-coating underlayer, which the company says will help reduce inflammation, platelet activation and aggregation and will help facilitate endotheliazation (or the healing of the vessel wall). The CALIX material is made of an inert, permanent and crack-proof coating, and covers the entire stent. The unique feature of the AXXION stent is its dual-CALIX layer. A first layer is applied to cover the entire stent, while a second layer is applied once the drug (paclitaxel) is applied to the ablumenal side. This second layer acts as a diffusion barrier to deliver the drug into the vessel wall. Once the drug is delivered, all that remains is the permanent CALIX film.
Sorin's JANUS stent is a new stent technology comprising microtrenches in the stent struts. These micro-trenches are filled with a drug called tacrolimus, and then a thin film of Carbofilm is applied to seal the trench. Once implanted, the film dissolves, releasing the drug into the vessel wall and leaving behind a bare metal stent.
Looking Forward
There are two DES technologies to look forward to. One is a novel stent technology that will allow physicians to stent longer and more complex lesions faster. The other is a next-generation bifurcation DES technology that approaches bifurcations from a completely different angle.
In contrast to other bifurcation technologies that are either currently available or in development, Cappella's Sideguard ostium-protection device is truly novel in concept and design.
“Previous bifurcated stent designs have failed to provide a deliverable and effective solution,” said Cappella's Guy Neev, vice president, Business Development. “Cappella is providing a new drug delivery platform that approaches the bifurcation challenge from a truly different angle.”
The company recently completed its proof-of-concept of the Sideguard stent in animals and is looking to start a DES program.
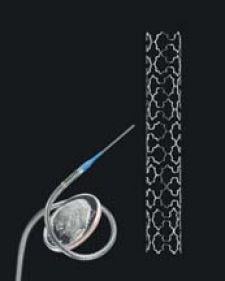
The Sideguard features a unique flared cap that naturally conforms to the side-branch ostium, which is one of the most problematic issues facing interventionalists today when trying to position a stent at the bifurcation.
And, unlike its stiffer bare-metal counterparts, which require high-pressure balloon deliveries, the Sideguard is made of nitinol, a nickel-titanium composite, which allows the stent to self-expand into place. This reduces the damage caused to the vessel wall during stent deployment, which can cause inflammation and platelet activation that can lead to stent thrombosis.
The most significant feature of the stent, besides its flared cap, is that the Sideguard has been specifically designed for placement in side branches. According to Neev, this is a significant advancement because once the stent is in place in the side branch, the interventionalist can then stent the main branch with little difficulty.
Xtent Inc. has developed a stent platform and DES system that will address limitations many first- and next-generation DES technologies have — namely the ability to match stent length to lesion length more accurately in situ. On January 5, 2006, Xtent released a statement that it had achieved two firsts: (1.) delivering two stents using one catheter and (2.) placing the longest stent ever.
Eberhard Grube, M.D., chief of Cardiology, Siegburg Heart Center in Siegburg, Germany, and principal investigator for Xtent's CUSTOM II clinical trial, was able to use the same catheter to deliver two stents of different lengths (28 mm and 32 mm) in separate arteries (left circumflex and the LAD). In another patient, Dr. Grube delivered a single, 52-mm stent, the longest stent ever placed in the coronary arteries from a single catheter.
Because of its unique design, the Xtent allows operators to customize and match stent lengths to lesions in situ. The CUSTOM II trial also serves as Xtent's DES system clinical trial. Xtent Inc. has licensed Biolimus A9 from Biosensors International.
Even though first-generation DES are not the silver bullet, they have, nevertheless, benefited millions, saving countless lives and sparing many patients from going under the knife. The fact is, every first-generation product has its problems, but as times goes on products improve as manufacturers make changes and adjustments to address them. Safety remains the biggest concern, but on the bright side options are appearing on the horizon.
Dr. Baim believes the future of DES lies with ablumenal coatings and pro-endothelial surfaces. He also says the development of specialized stents, such as Cappella's Sideguard ostium-protection device will be a very important advancement in stent technology. Most importantly, he said, “all [interventionalists] want are drug-eluting stents that are as easy to deliver as current balloon catheters and available in the same size range as current balloons.”


 July 02, 2024
July 02, 2024 









