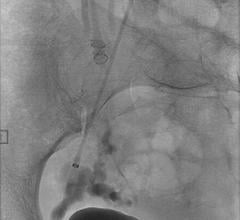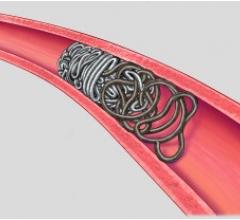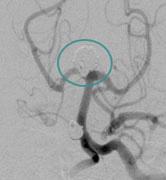
April 8, 2011 – The U.S. Food and Drug Administration (FDA) has approved an aneurysm treatment system as a Humanitarian Use Device (HDE). NeuroVasx’s cPAX Aneurysm Treatment System can be used to treat large, giant and wide-neck cerebral aneurysms, which are typically the most difficult to treat.
The HDE allows for the treatment of up to 4,000 patients per year in the United States.
"The large, giant and wide-neck cerebral aneurysm population continues to remain the most challenging to treat,” said Christopher Dowd, M.D., clinical professor of neuro-interventional radiology at the University of California at San Francisco and medical director for NeuroVasx. “cPAX will offer physicians an alternative solution that we believe can make a significant impact in treatment and outcome for these patients."
"The longer term stability we have seen in the clinical studies using cPAX in larger aneurysms gives me great confidence in the positive impact this product will have on the care of our patients,” said Ricardo Hanel, M.D., Ph.D., associate professor of neurosurgery at the Mayo Clinic in Jacksonville, Fla., and co-principal investigator in the cPAX Clinical Trial.
A cerebral aneurysm is an abnormal bulge or sac in the wall of an artery in the brain which can be caused by a number of factors including congenital defects, high blood pressure, atherosclerosis, cancer, drug use or head trauma. If a cerebral aneurysm ruptures, it can lead to a hemorrhagic stroke, or bleeding on the brain.
According to the National Institutes of Health, approximately 40 percent of these patients do not survive the first 24 hours. The worldwide incidence of cerebral aneurysms is estimated to be 320,000 annually, approximately 200,000 of which may be treatable with intracranial surgery using minimally invasive techniques.
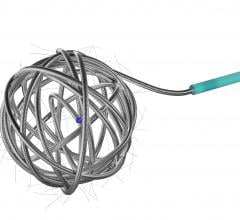
cPAX is a polymeric strand delivered into the aneurysm using a technique similar to platinum coil technologies. Because of its soft polymeric material, the device is designed to achieve more complete filling of the aneurysm with the probable benefit of greater long-term stability. A significant feature is that it offers the physician the ability to detach the device at any point. The polymeric material also allows for non-invasive CT and MRI scans with little or no artifact for more accurate patient follow-up assessment.
For more information: www.neurovasx.com


 June 05, 2025
June 05, 2025