February 24, 2020 — The U.S. Food and Drug Administration (FDA) approved Esperion's bempedoic acid (Nexletor) tablet, an oral, once-daily, non-statin low-density lipoprotein cholesterol (LDL-C) lowering medicine. The drug is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.
The effect of bempedoic acid on cardiovascular morbidity and mortality has not been determined. The agent is the first oral, once-daily, non-statin LDL-C lowering medicine approved since 2002 for indicated patients.
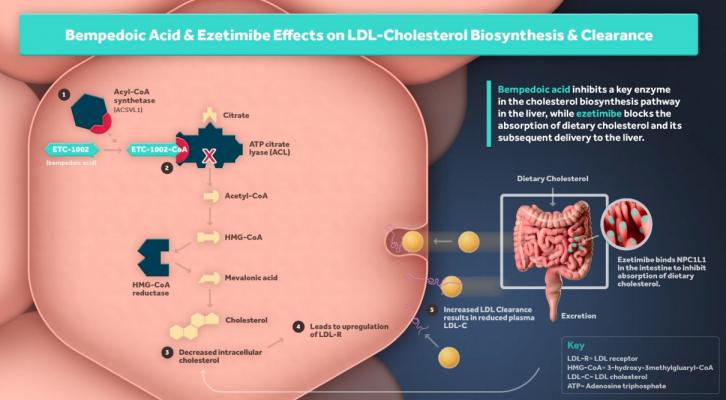
Bempedoic acid is a first-in-class ATP Citrate Lyase (ACL) inhibitor that lowers LDL-C by inhibition of cholesterol synthesis in the liver.
“The FDA approval of Nexletor provides an important option for patients living with elevated LDL-C and ASCVD or increased risk for cardiovascular disease because of HeFH,” said Christie M. Ballantyne, M.D., chairman of Esperion’s Phase 3 Executive Committee and professor and chief of cardiology at Baylor College of Medicine in Houston. “There are millions of patients who are unable to reach their LDL-C targets despite available medicines. Nexletor is the first oral, once-daily, non-statin treatment option for indicated patients in nearly two decades.”
FDA Approval of Nexletor Based Two Clinical Studies
The approval of bempedoic acid is supported by a global pivotal Phase 3 LDL-C lowering program conducted in more than 3,000 patients. In these studies, Nexletor provided an average of 18 percent placebo corrected LDL-C lowering when used with moderate or high intensity statins. Results from the Phase 3 development program have been published in The New England Journal of Medicine[1]and The Journal of the American Medical Association.[2]
Nexletor is a first-in-class ATP Citrate Lyase (ACL) inhibitor that lowers LDL-C by reducing cholesterol biosynthesis and up-regulating the LDL receptors. Completed Phase 3 studies conducted in more than 3,000 patients, with over 2,000 patients treated with Nexletor, demonstrated an average 18 percent placebo corrected LDL-C lowering when used in patients on moderate or high-intensity statins. The pharmaceutical is the first oral, once-daily, non-statin LDL-C lowering medicine approved in the U.S. in nearly 20 years for patients with ASCVD or HeFH.
Bempedoic acid was generally well-tolerated in clinical studies. Label warnings and precautions include hyperuricemia, with the development of gout in a small percentage of patients, as well as increased risk of tendon rupture or injury. Overall in Phase 3 studies, the adverse events reported most frequently in patients who received Nexletor were generally reported at similar rates in patients who received placebo. The most common adverse events reported with Nexletor (incidence ≥ 2% and greater than placebo) were upper respiratory tract infections, muscle spasms, hyperuricemia, back pain, abdominal pain or discomfort, bronchitis, pain in extremity, anemia, and elevated liver enzymes. The majority of adverse events reported with Nexletor were mild to moderate in severity and balanced in occurrence with adverse events in patients receiving placebo.
For additional information, please see full prescribing information at Esperion.com.
Nexletor Will be Launched in U.S. Market in Spring 2020
Esperion said it plans to launch Nexletor commercially in the U.S. March 30, 2020.
Esperion said it is working with health insurance providers to help ensure broad insurance coverage and patient access to Nexletor. Eligible patients with commercial drug insurance coverage for the new agent may pay as little as $10 per fill, up to a three-month supply, the company said. Additionally, Esperion said it is committed to achieving the lowest branded tier coverage for Medicare patients. Esperion will provide resources to patients whose physician recommends treatment with Nexletor. These resources include educational materials, a dedicated call center, as well as a co-pay program for eligible patients.
Esperion’s second LDL-C lowering medicine, the bempedoic acid/ezetimibe combination tablet, is currently under review by the U.S. FDA.
Bempedoic acid is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL-C. The effect of bempedoic acid on cardiovascular morbidity and mortality has not been determined.
CLEAR Cardiovascular Outcomes Trial Evaluating Bempedoic Acid
The effect of bempedoic acid on cardiovascular morbidity and mortality has not been determined. Esperion initiated a global cardiovascular outcomes trial (CVOT) to assess the effects of bempedoic acid on the occurrence of major cardiovascular events in patients with, or at high risk for, cardiovascular disease (CVD) who are only able to tolerate less than the lowest approved daily starting dose of a statin and are considered "statin averse." The CLEAR Cardiovascular Outcomes Trial is an event-driven, global, randomized, double-blind, placebo-controlled study that completed enrollment in August 2019 of 14,032 patients with hypercholesterolemia and high CVD risk at over 1,400 sites in 32 countries.
What is LDL Cholesterol?
Low-density lipoprotein cholesterol (LDL-C) is a waxy, fat-like substance that’s found in the body. Elevated LDL-C contributes to a buildup of this fat in the arteries and can lead to cardiovascular events including heart attack and stroke. Despite standard of care treatments, including statin therapy, it is estimated nearly 15 million patients (approximately one in four patients) in the U.S. cannot achieve guideline recommended LDL-C levels.
References:


 January 28, 2026
January 28, 2026 









