First Patient Enrolled in Study to Detect, Treat Vulnerable Plaques Suspected to Cause Heart Attacks
July 3, 2014 — Infraredx Inc. announced the first patient enrolled in PROSPECT II, a multi-center, prospective clinical study designed to assess the ability of intravascular imaging to identify non-flow obstructing vulnerable plaques. Lipid core plaque (LCP), which is suspected to be vulnerable plaque, is a type of fatty coronary artery plaque implicated in most heart attacks, and will be identified using the Infraredx TVC Imaging System, a first-in-class dual modality intravascular imaging system. The study’s first patient was enrolled by David Erlinge, M.D., Ph.D., one of the study’s principal investigators, from Lund University in Sweden.
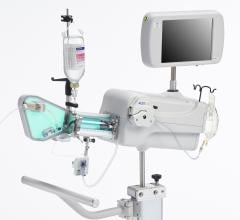
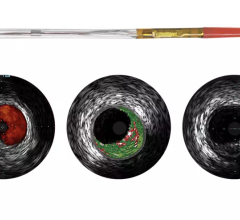
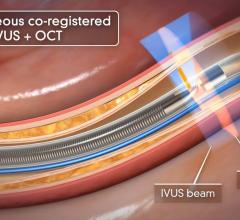
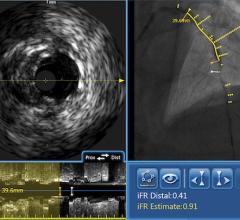
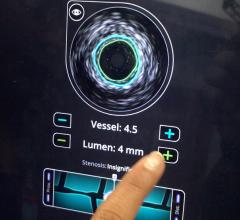
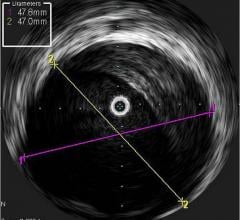
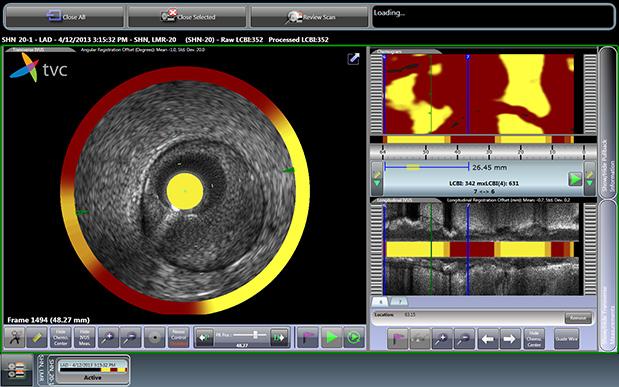
The TVC Imaging System is the only device approved by the U.D. Food and Drug Administration (FDA) to detect the presence of LCPs. The technology integrates near-infrared spectroscopy (NIRS) to detect LCPs, with enhanced intravascular ultrasound (IVUS) to visualize the vessel structure, and is used to guide percutaneous coronary interventions (PCI).
“There is mounting clinical evidence pointing to the role of lipid-rich plaque as the main cause of heart attacks,” said Erlinge. “The PROSPECT II study will allow us to test our hypotheses that NIRS-IVUS imaging can identify the vulnerable plaques that cause heart attacks, and that preemptive treatment of the most dangerous plaques with PCI can prevent the development of further narrowing of the coronary arteries, as well as plaque erosion and rupture.”
Infraredx will provide the primary funding for the study, with additional support from The Medicines Co. and Abbott Vascular.
In 2011, the New England Journal of Medicine published results from the original PROSPECT study, which was the first to prospectively demonstrate that vulnerable plaques can be identified through imaging techniques months to years before adverse events occur. The PROSPECT II study, which will enroll 900 patients at 16 leading cardiac catherization laboratories across Scandinavia, will use the TVC Imaging System to identify vulnerable plaques in the three major coronary arteries and follow patients for at least three years to detect the occurrence of coronary events. In addition, 300 patients with “bulky” plaques (detected by intravascular ultrasound), which have been shown to be at high risk for causing future adverse events in the first PROSPECT study [1], will be randomly assigned to treatment with Abbott’s Absorb Bioresorbable Vascular Scaffold (BVS) or optimal medical therapy.
Absorb is a first-of-its-kind device which is under investigation in the United States, and received CE mark in 2011. Absorb functions similarly to a stent and is designed to open a blocked vessel and restore blood flow, but because it is made from bioresorbable materials similar to dissolving stitches, it dissolves completely over time.[1] Absorb is called a scaffold to indicate its temporary nature. In data from international studies, regression of plaque has been observed at the site where Absorb has been implanted, a unique effect not typically seen with permanent metallic stents. Data from this sub-study, termed PROSPECT-ABSORB, will be analyzed in patients with and without a cholesterol signal at the site of the large plaque, making this the first large-scale study of the identification and preventive treatment of vulnerable plaques.
The PROSPECT II study was prompted by promising new data on the ability of the TVC Imaging System to detect large vulnerable plaques precisely at the coronary artery sites at which an ST-segment elevation myocardial infarction (STEMI) occurred causing a dangerous type of heart attack.
PROSPECT II is an investigator-initiated study led by co-principal investigators Gregg W. Stone, M.D., professor of medicine, Columbia University Medical Center and co-director, Medical Research & Education Division at the Cardiovascular Research Foundation in New York; and David Erlinge, M.D., Ph.D., director of the Department of Cardiology, Lund University, Skane University Hospital in Lund, Sweden. The Cardiovascular Research Foundation (CRF) will oversee the study and provide angiographic and intravascular imaging data core lab analysis and biostatistical analysis. The Uppsala Clinical Research Center will organize and coordinate the clinical management of the trial, including patient recruitment, monitoring and data collection. Patients will be recruited through the Swedish and Norwegian Coronary Angiography and Angioplasty Register (SWEDEHEART) and the corresponding register in Denmark.
“The success of PROSPECT II would be a major step toward the goal of prospective identification and eventual treatment of the vulnerable plaques causing unanticipated coronary events,” said Stone. “Thrombosis of vulnerable plaque is the leading cause of cardiac death, myocardial infarction and heart failure, which are debilitating and costly conditions. We look forward to implementing this groundbreaking study, which has the potential to change how we approach patients with coronary artery disease.”
For more information: www.infraredx.com
References
1.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235


 September 18, 2025
September 18, 2025