First Robotic-Assisted STEMI Stent Performed at Sanford Aberdeen Medical Center
July 25, 2013 — Sanford Aberdeen Medical Center in Aberdeen, S.D. became the first hospital to perform a robotic angioplasty for a patient with an acute heart attack, achieving a far better door-to-balloon time than the national standard. Interventional cardiologist Puneet Sharma, performed the percutaneous coronary intervention (PCI) to treat a patient that had experienced a heart attack and presented to the Sanford Aberdeen emergency department. Utilizing the U.S. Food and Drug Administration (FDA)-cleared CorPath System, Sharma was able to perform the robotic-assisted angioplasty procedure and restore blood flow to the patient’s heart within 68 minutes of their arrival.
The current nationwide standard for door-to-balloon time — from when a patient with a heart attack is presented to the emergency department (ED) to inflating a balloon in his coronary arteries to restore blood flow to the heart — is 90 minutes. These standards were developed by the American College of Cardiology (ACC) to improve timely access to the cath lab and by that improving clinical outcome.
“Timely access to emergency cardiac care and survival is partly dependent on access to services and technology,” said Sharma. “Being able to perform a CorPath Robotic Angioplasty on a STEMI patient within 68 minutes is a great benefit. As shown with the latest procedure, robotic-assisted angioplasties improve rural access and quality of care as more patients in this area will have access for advanced specialty care. The implementation of the CorPath System and its ability to precisely and rapidly execute an angioplasty procedure with only one stent in the patient heart is a great example of Sanford Health’s commitment to enhanced clinical outcome for our patients.”
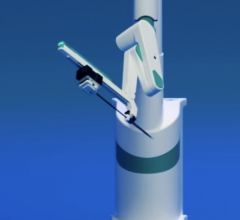
The CorPath System is the first and only FDA cleared technology that enables precise, robotic-assisted angioplasties to open arteries and restore blood flow in patients with coronary artery disease. Seated in an interventional cockpit, the interventional cardiologist advances stents and guidewires via a joystick with millimeter-by-millimeter precision. The system, which is quickly being adopted as the new standard of care in coronary angioplasty procedures, may also improve clinical outcomes by enabling precise measurement of the anatomy, which could potentially lead to better stent placements.
Sanford Health performs robotic-assisted angioplasty procedures with the CorPath System at both Sanford Heart Hospital in Sioux Falls and Sanford Aberdeen Medical Center. It is the first and only health system in the region utilizing the CorPath System.
For more information: www.corindus.com


 January 27, 2026
January 27, 2026