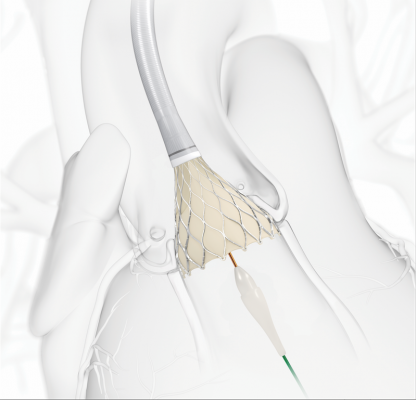
August 4, 2016 — Medtronic announced CE (Conformité Européenne) mark market clearnace for the self-expanding, recapturable and repositionable CoreValve Evolut R System to treat aortic stenosis patients who are at intermediate risk for open-heart surgery. The Evolut R System is the first transcatheter aortic valve replacement (TAVR or TAVI) device to obtain this expanded indication in Europe.
"The unique design of the self-expanding, supra-annular Evolut R System, coupled with its ability to be recaptured and repositioned for accurate valve placement, enables this device to be a viable treatment alternative for patients at intermediate surgical risk," said Prof. Eberhard Grube, M.D., director of the Structural Heart Program at University Hospital in Bonn, Germany. "The highly-anticipated intermediate risk indication marks an important milestone for the industry as we look to safely expand TAVI access to younger and less sick patient populations."
The new intermediate risk indication approval for the CoreValve Evolut R was based on positive clinical data from the Nordic Aortic Valve Intervention (NOTION) Trial and from a subset analysis from the CoreValve U.S. High Risk Pivotal Trial. Data from the NOTION trial showed that comparable clinical outcomes to surgery can be achieved by using CoreValve in patients who are good surgical candidates. Both datasets demonstrated excellent clinical performance for the CoreValve System with lower rates of all-cause mortality and major stroke compared to surgery. Additionally, data showed low incidences of procedural complications and superior hemodynamic performance (blood flow) compared to surgery.
The Evolut R valve is delivered through the EnVeo R Delivery Catheter System, which features an InLine Sheath that significantly reduces the profile to the lowest currently on the market (14 French equivalent, less than 1/5 inch). A smaller profile size provides a greater opportunity to treat patients with smaller vessels through the preferred transfemoral access route, and may minimize the risk of major vascular complications in some patients.
The CoreValve Evolut R System and the EnVeo R Delivery Catheter System are now approved for use in patients at extreme, high and intermediate surgical risk in Europe and other countries that recognize the CE mark. The CoreValve Evolut R System was FDA-approved for commercial use in the United States in June 2015 for severe aortic stenosis patients who are at high or extreme risk for surgery.
The CoreValve Evolut R System is not approved to treat intermediate risk aortic stenosis patients in the U.S.
Watch the video, "CoreValve Trumps Surgical Valve Replacement," and interview with Michael Reardon, M.D., professor of cardiothoracic surgery at DeBakey Heart and Vascular Center, and chairman of the patient screening committee, CoreValve U.S. pivotal trial
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world.
For more information: www.medtronic.com


 November 14, 2025
November 14, 2025 









