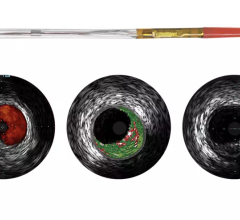
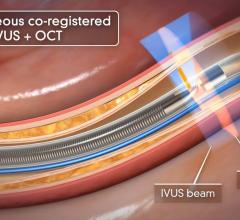
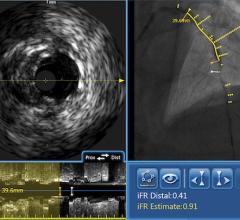
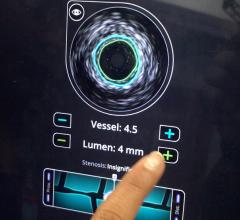
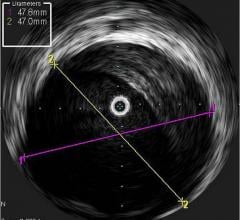
A major issue in treating chronic total occlusions (CTOs) is the lack of angiographic visualization. The flow of blood and contrast is completely blocked, eliminating the ability to navigate the vessel or see where wires are being poked. Not being able to see what you are treating is a big reason why operators do not attempt these lesions.
“All you see is the start and the end of the lesion. It can be very terrifying,” said William Lombardi, M.D., FSCAI, medical director of cardiac cath labs at St. Joseph Hospital, Bellingham, Wash.
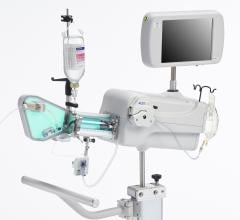
In a comparison of items needed to treat CTOs, Scott Huennekens, president and CEO of Volcano Corp., said to build a car you need wheels, steering and headlights. There are already interventional tools available to steer and treat CTOs, but operators still need a way to see where they are driving. “You are always driving in the dark. You have wheels and steering, but you have no headlights, so you can’t see where you are going,” Huennekens said.
Volcano is developing two forward-looking intravascular ultrasound systems (FL-IVUS). One is for visualization ahead of the catheter and the second will incorporate a radio frequency (RF) ablation tip.
Aaron Grantham, M.D., FACC, Mid-America Heart Institute, Kansas City, Mo., is not convinced that incorporating the RF tip will completely solve CTO treatment issues. He said prior RF catheters were unsuccessful. However, he thinks FL-IVUS has the potential to make visualizing and traversing CTOs with wires easier and safer. He is very interested to hear reports about the first-in-man experience scheduled to start this fall in the Netherlands and Chile. “Translation from bench models to bedside will be a major milestone for FL-IVUS,” he said.
Volcano hopes for European CE-mark clearance of its FL-IVUS device by mid-2011, and in the U.S. by the end of 2011. The company said its first-in-man trial for the FL-IVUS ablation system is set to begin by mid-2011.
“In theory, forward-looking IVUS has promise. However, some operators already have a 95 percent success rate, so it will have to show it can do the procedure faster,” Lombardi said. “All technology is going to do is shorten the procedure.”


 September 18, 2025
September 18, 2025