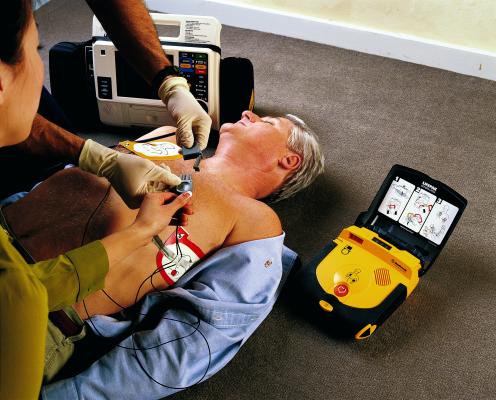
A Physio-Control LifePak CR Plus defibrillator shown in use during a hand-off to paramedics, who use larger, more sophisticated defibrillator-monitors (behind gloved paramedic).
Automated external defibrillators (AED) are portable and lightweight devices used to deliver an electric shock through the chest to the heart to bring irregular rhythm of the heart back to normal in patients with sudden cardiac arrest (SCA). In addition, AEDs help in detecting and identifying life-threatening arrhythmias. Almost 95 percent of patients with SCA die within a minute, which has led to increase in the demand for AEDs.
AEDs aid in responding to a medical emergency, especially by the non-medical personnel such as flight attendants, police, fire service personnel, security guards and other lay rescuers, who have been trained properly to use AEDs. Therefore, AEDs are used at public places such as airports, shopping centers, schools, hotels, offices, doctor’s office and home. The increasing installations of public access AEDs, growing incidence of cardiovascular diseases and growing awareness regarding the lifesaving potential of AEDs have fueled the market growth. AEDs dominate the world defibrillator market due to their user-friendly feature and ability to save a patient’s life during emergencies.
In the public access market, defibrillators are used in schools, stadiums, casinos, airports, corporate offices, government and community centers, hotels, restaurants, transportation and health clubs, among others.
AED Technology Advances
Competitors in the market are launching technologically advanced defibrillators for gaining a competitive edge. For instance, Defibtech expanded its line of dual language AEDs so that citizens of the 40 countries, where the Defibtech’s products are exported, can easily use the product. A home use defibrillator has been introduced in the AED market and is estimated to offer productive opportunities in the near future. Moreover, in July 2015, Defibtech shipped more than 250,000 AEDs, making a remarkable achievement of saving hundreds of lives by making AEDs easily available.
Philips HeartStart, approved as a home-use defibrillator, is a success in the AED market, and its increasing use among patients suffering from irregular heartbeat is projected to provide massive opportunities in the near future. However, lack of awareness about SCA and use of AEDs at public places and product recalls are the major factors hindering the growth of the world AEDs market.
AEDs are designed in a way to make them useful to both life-support trained personnel and the general public. Newer AEDs guide the user by either giving signals (such as pre-recorded verbal instructions) to the rescuer to deliver shock or the system will automatically deliver the shock. The Zoll AED Plus is the only AED that is presently coaching the rescuers to deliver the correct rate and depth of the compressions using an accelerometer that is built in the electrode pad.
Smartphones to Locate the Nearest AED
While there are many AEDs in public places, during an emergency there locations may not be known to the general public. Some vendors have developed apps to help rapidly locate the nearest AED to speed their deployment. Key market players include HeartSafe AED Locator and Arrhythmia Alliance, both of which invite any medical practitioner, individual person, school, community, business and sports facility to register their AEDs.
In addition, Some communities have also created their own programs to help speed connecting public first responders and AEDs with patients in SCA. Seattle created a program where residents can download an app on their phones that alerts them to a SCA 911 call near them, which opens a map showing their location and the location of registered AEDs in the immediate area. East of England Ambulance Service in the United Kingdom, part of the National Health Service (NHS) recently launched “Their Life, Your Hands” campaign to make people aware about the public access defibrillator located to their nearest community. When AEDs are housed in the cabinets and installed in the community, they are known as community public access defibrillators (CPAD).
Market Drivers
Recommendations for the usage of automated external defibrillators at public places by various cardiology and medical societies has helped rapidly increased the adoption rate in developed economies.
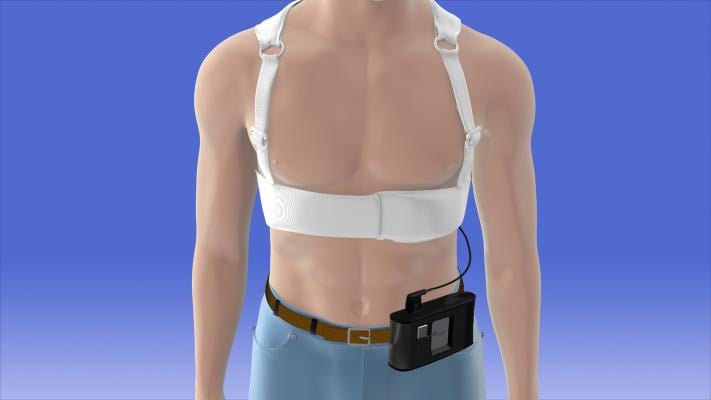
There also is expected growth in the market for wearable cardioverter defibrillator (WCDs), which are used by patients as a temporary alternative or to bridge to a long term implantable cardioverter defibrillator (ICD) implantation.
Editor's note: This article was compiled by market researchers at Allied Market Research, a global market research and business consulting wing of Allied Analytics LLP based in Portland, Ore. www.alliedmarketresearch.com


 January 13, 2026
January 13, 2026 









