Maquet recently introduced a 50 cc balloon for its IABP system.
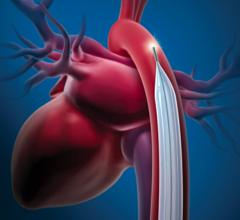
The intra-aortic balloon pumps (IABPs) have been the gold standard for minimally invasive circulatory support for 40 years, but they were joined in recent years by percutaneously deployed ventricular assist devices (P-VADs).
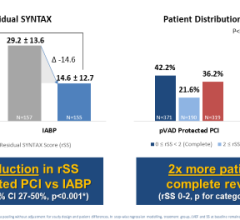
IABP makers Teleflex and Maquet claim their devices offer between .5 to 1 liter of augmentation flow per minute (lpm). P-VAD makers Abiomed and CardiacAssist claim between 2.5 and 5 lpm. However, the higher VAD flow rates come at higher cost ($800-$1,200 for an IAB as compared to $22,000 to $35,000 for a P-VAD), and are more technically challenging to deploy.
Industry experts say it is unlikely IABPs will be replaced with percutaneous VADs anytime soon, mainly due to cost. Less than 3,000 P-VADs have been used since their U.S. introduction, while it is estimated that 130,000 IABPs are used annually in the United States.
“I think the balloon pump will remain the best device with the biggest bang for the buck. It is always going to be the first thing that gets done,” said CardiacAssist Inc. President and CEO Michael Garippa. “We really come in after the balloon pump.” He said P-VADs are generally employed only after a patient receives inotropes and an IABP, but need more support. Garippa said these patients are primary drivers for increased P-VAD use.
Michael R. Minogue, Abiomed CEO, president and chairman of the board, believes his company’s Impella p-VADs should be used as a front-line device. “With unstable patients, the best thing you can do is increase output, and balloon pumps don’t provide that support,” Minogue said. “Fifty-eight percent of our patients who are getting the Impella also had a balloon pump first that did not deliver the support they needed.”
“There is a segment of the critically ill cardiac patient population that remains hemodynamically unstable even with an IABP and need a VAD. The VAD is the next step after an IABP,” said Tom Mahoney, Teleflex Medical’s global clinical marketing manager.
“VADs are very much a niche market right now,” said Julio Monroy, Maquet’s global product manager, cardiovascular systems, adding VADs appear to work well in some highly specific indications, such as high-risk PCI.
“The question is, ‘what is best for the patient and what tool should you use?’” Garippa said.
IABPs Technology Improves
IABPs use counterpulsation to augment blood flow by inflating during diastole and unload myocardial work by deflating during the isovolumetric phase of systole. “The issue that comes up is with irregular heart beats,”
Mahoney said. Unstable patients with irregular rhythms and variable pulse pressures require immediate adjustments.
He said Teleflex’s AutoCAT 2 WAVE IABP uses the physiologically-based WAVE algorithm that provides real-time adjustments, even for atrial fibrillation.
The development of IAB catheters with fiber optics
allow for real-time aortic pressure assessments and the speed of signal transmission can be translated into real-time data for immediate balloon adjustments, Mahoney said.
“The systems are designed to measure and compensate for irregular heart rates,” Monroy said. He said Maquet’s CS100 with IntelliSync offers one-button start up and automatically adapts to changing conditions.
Limb ischemia was an issue due to the large IABP catheters used until this decade. Today’s 7 and 8 French catheters can sometimes be used without an introducer sheath and have greatly reduce bleeding complications associated with the larger catheters. However, Monroy said bleeding complications and limb ischemia can still be an issue with P-VADs, which use large catheters and introducers to
accommodate components between 9 to 21 French.
In 2008, Maquet initiated the CRISP-AMI trial to evaluate 300 randomized patients to see if IABP use reduces infarct size and increases myocardial salvage in high-risk acute myocardial infarction (MI) patients. It also sponsored the BCIS-1 trial, which examines IABP use in high-risk PCI. The study includes 300 patients divided between rescue PCI and elective PCI. Monroy said 12-18 month outcomes from the studies are expected in 2010.
P-VAD Use,/strong>
Abiomed and CardiacAssist both reported record sales in 2009, showing a big interest in P-VADs. The biggest differences between the Abiomed Impella 2.5 and 5.0 devices and CardiacAssist’s TandemHeart boil down to cost, set- up time, and ease of use.
Abiomed wants to prove the merits of Impella and created a registry and is sponsoring several clinical
trials. Data on 300 patients from the USpella Registry is expected at ACC 2010. A key trial underway randomizes high-risk PCI patients to Impella or IABPs. Minogue said results should be available in 2012.
References on Device Performance:
American Heart Journal. 2003; 145: 700-707
Hellenic Journal of Cardiology. 2008; 49: 382-387
EuroIntervention. 2006; 2: 84-90
EuroIntervention. 2006; 2: 91-100
Annuals of Thoracic Surgery. 2005; 79: 1017–1022
Annuals of Thoracic Surgery. 2005; 79: 872– 880
American Heart Journal. 2006; 152: 469-469
American Journal of Cardiology. 2006; 97: 990-992
JACC, Cardiovascular Interventions. 2009; 2: 91-

 August 14, 2023
August 14, 2023