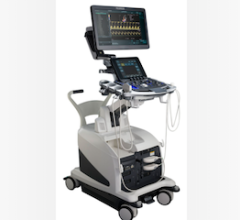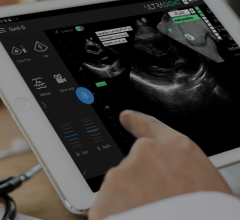
ICE is offered on GE Healthcares Vivid i and Vivid q portable ultrasound systems. Images can be integrated into GEs CardioLab electrophysiology system.
Intracardiac echocardiography (ICE) offers a clearer view of transcatheter devices and electrophysiology (EP) procedures from inside the heart.
Angiography has limits to guiding many cardiac therapies because X-ray fluoroscopy does not clearly visualize soft tissue. It also exposes patients to ionizing radiation. ICE can be used to better visualize intracardiac structures and intracardiac devices during procedures. It is also useful in transseptal catheterization, as an aid in EP mapping, pulmonary vein isolation, and complex arrhythmia ablation. ICE also helps guide septal defect and left atrial appendage closure devices. In addition, it can visualize intracardiac thrombus, assess ablation lesion characteristics and monitor interactions between devices and the tissue, such as tenting of the fossa ovalis. This increased visualization inside the heart contributes to greater clinical confidence, fewer complications, expanded treatment capabilities, and improved workflow.
Key Players
ICE catheters and systems are offered by Siemens, Boston Scientific, Biosense Webster, St. Jude Medical, and GE Healthcare.
In 2008, St. Jude Medical purchased EP MedSystems for about $92 million to expedite its entry into the ICE market. The ViewMate II ICE system is manufactured for St. Jude by Philips. St. Jude specializes in the EP market where ICE is used extensively.
Another EP giant with ICE technology is Biosense Webster. It partnered with Siemens and GE Healthcare to interface their ICE systems with its own EP navigation and ablation treatment systems. Biosense Webster also is the exclusive provider of Siemens’ AcuNav ICE catheter. In addition, the company began offering its own ICE catheter in 2009.
Systems and Catheters
Siemens was an early developer of ICE, introducing its AcuNav ICE catheter in 1999. Since then it has sold more than 100,000 catheters worldwide. The single-use AcuNav comes in 8 and 10 French sizes and offers detailed resolution and penetration down to 15 cm in the heart. The user determines the desired trade off between resolution and penetration by choosing from up to four imaging frequencies. The catheter is compatible with the Siemens’ Acuson Sequoia, Aspen and Cypress and CV70 echocardiography systems.
Boston Scientific offers the Ultra ICE catheter for use with its iLab system, which also provides intravascular ultrasound (IVUS) imaging capability. The Ultra generates a panoramic 360-degree image perpendicular to the catheter, with the tip as a central reference point. This allows the user to visualize structures (such as the fossa) directly adjacent to the catheter tip and still see a detailed cross section of the entire septum.
Introduced in the fall of 2008, GE Healthcare began offering ICE as an option on its compact Vivid i and Vivid q ultrasound systems. The systems use the AcuNav ultrasound catheters.
Biosense Webster introduced the SoundStar 3D ICE catheter in 2009. The 90 cm, 10 French catheter has a similar construction to the AcuNav. It has the addition of a sensor in the tip, which provides location information to the Carto EP XP Navigation System.
The St. Jude Medical ViewMate II ultrasound system uses the ViewFlex Plus ICE catheter. It provides stability and control over viewing angles and positioning. The catheter has a large curvature radius, steering angles to 120-degrees, torsional control, and a simple one-handed control mechanism. The system has intuitive on-screen menus, operates in modes for 2-D high-resolution imaging of cardiac structures, color blood flow Doppler, pulsed and continuous wave spectral Doppler for quantification of blood flow, and M-mode imaging. The system also is configured for transesophageal echo (TEE), transthoracic echo (TTE) and digital imaging and communications in medicine (DICOM) capabilities.
New ICE Technology
Siemens demonstrated the first volume ICE (VICE) catheter at the 2009 Scientific Sessions of the Heart Rhythm Society. The Acuson AcuNav V catheter offers real-time volumetric information to improve therapeutic ablation procedures and other EP applications. Adding the benefits of volume imaging to ICE will help improve and simplify the visualization of ablation catheter positioning, catheter-tissue contact and bubble formation during radiofrequency energy delivery. The technology also eliminates the need to “stitch” together multiple scans to create a single image.
The AcuNav V volume catheter is not yet cleared by the U.S. Food and Drug Administration (FDA). It will be available for use only with the Acuson SC2000 volume imaging ultrasound system.

 October 09, 2025
October 09, 2025