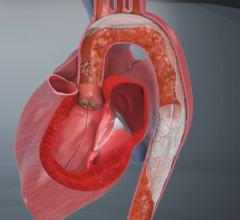
The use of embolic protection devices (EPD) during percutaneous cardiac procedures has helped reduce the number of complications due to debris being released into the bloodstream and causing blockages in smaller vessels. The devices are designed to capture and remove debris that may be dislodged during procedures.
There are three classes of EPD devices:
1. Distal occlusion devices use a balloon catheter to temporarily block the flow of blood in a vessel while the intervention is performed. Any debris trapped in a stagnant column of blood can be removed through an aspiration catheter prior to the distal occlusion being released. Experts say one disadvantage of distal occlusion is that the end organ is ischemic during the time the device is in use. This means use of these devices requires quick action by the interventional team to complete a procedure in four to six minutes so blood flow can be restored.
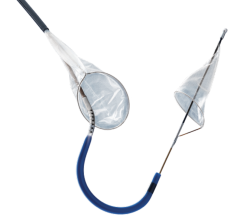
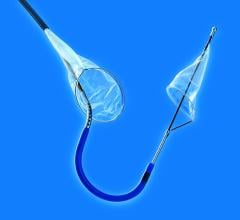
2. Distal filters have a small net, referred to as a “basket,” which is supported by an expandable metal. The device allows blood to continue circulating through the pores of the filter. These filters can be used for up to five to 10 minutes, buying additional time for a procedure.
Most devices have filter pore sizes of around 100 microns. In comparison, a white blood cell measures about 25 microns. Devices that use a nitinol metal mesh filter only tend to let bigger particles through, up to about 150 microns in size. Experts say pores smaller than 100 microns can easily become clogged with platelets.
Distal filters also allow for contrast media injection during the procedure for lesion and arterial visualization.
At the beginning of the procedure the filter is constrained inside a delivery sheath, which the physician guides into position past the area of blockage to be treated. Once in place, the constraint is released and the filter pops open into position. If plaque or blood clots are dislodged during the procedure, the filter will catch them to prevent the emboli from traveling downstream, reducing the chance of an event such as a heart attack. At the end of the procedure the filter containing debris is captured in a retrieval sheath and removed from the patient’s body.
The diameter of the catheters used in placement and retrieval of the protection devices are referred to as the product’s profile.
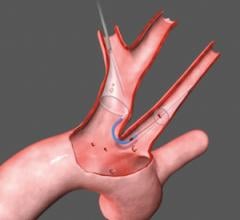
3. Proximal occlusion devices interrupt the flow of blood upstream of the blockage. Proximal occlusion balloons are placed before the lesion is accessed and are the only type of device that allows crossing of the stenosis under protection.
Physicians say the key factors in choosing EPDs are their ability to maintain perfusion, be effective in capturing emboli, a low profile, deliverability, retractibility and the ability to recover all the debris collected.
Visualization of the protection device using imaging techniques is important because the device may not be seated well in the vessel, which could allow debris to flow around it. This may take views from multiple angles to determine proper positioning. Clinicians can also check to make sure the protection device is situated in the right location.
Experts say prolonged use of distal embolic protection devices can be associated with slow-flow or no-flow, particularly with soft, large plaques. Rapid deployment of an EPD and execution of a procedure by an experienced staff is critical to improving patient outcomes, physicians say.
While most EPDs are cleared by the FDA for coronary saphenous vein grafts interventions and carotid artery stenting, doctors frequently use them for off-label percutaneous procedures to protect patients from embolisms.


 April 25, 2023
April 25, 2023