May 12, 2014 — Datascope Corp./Maquet initiated a voluntary worldwide field correction of some of its intra-aortic balloon pumps (IABPs) due to a potential mechanical failure of the fan assembly associated with the power supply. All customers that may have an IABP affected by this field correction have been notified. The company says there are about 12,360 affected units sold globally.
The firm received 106 reports of device malfunctions, which caused one death, involving its system 98/98XT, CS100, CS100i and CS300 IABPs. The firm determined that the fan assembly for the affected IABPs could contain a misshapen retaining ring. This retaining ring could separate within the fan assembly, causing the fan to stop rotating. This could result in the power supply overheating and the IABP would shut down without any warning. This device failure would cause the balloon to stop inflating and deflating. It may result in decreased blood flow to the heart and the rest of the body, difficulty in weaning the patient from cardiopulmonary bypass, and clotting or blockage of blood vessels to the intestines, kidneys, or legs. The possible long range consequences of this include organ injury or tissue damage, possibly leading to patient death.
The recalled systems were sold under the Datascope Corp. and include system 98/98XT (part numbers: 0998-00-0446-xx, 0998-UC-0446-xx, 0998-00- 0479-xx and 0998-UC-0479-xx), CS100/CS100i (Part Numbers: 0998-00-3013-xx, 0998-UC-3013-xx, 0998-UC-0446Hxx and 0998-UC-0479Hxx) and CS300 (part numbers:0998-00-3023-xx and 0998-UC-3023-xx).
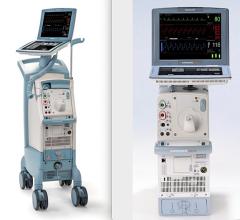
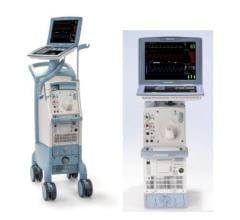
The IABP is an electromechanical system used to inflate and deflate an intra-aortic balloon to provide temporary support to the left ventricle via the principle of counter pulsation.
Between Jan. 1, 2003, and June 30, 2011, specific System 98/98XT, CS100/CS100i and CS300 IABPs were manufactured with an affected fan assembly, or may have received an affected fan assembly during an upgrade/service of the IABP in the field. The affected IABP units were distributed in the United States and worldwide (in more than 100 countries). The affected IABP's can be identified by part and serial number. Each Maquet service representative has a list of the affected serial numbers and will check each affected part during the corrective action.
The affected system 98/98XT, CS100, CS100i and CS300 IABPs involved in the field correction can be used while waiting for parts and service. Customers should adhere to the instructions for use when using an affected IABP.
The U.S. Food and Drug Administration (FDA) has classified this action as a Class 1 recall. FDA defines Class 1 recalls as, "a situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death." To date, there have been no reported patient injuries or deaths related to the power supply malfunction.
The corrective action associated with this recall is to provide a replacement fan assembly to all IABPs containing an affected fan assembly. A Maquet service representative will contact those facilities with affected IABP's to schedule corrective action and document this corrective action during a visit to the customer.
For additional information regarding this field correction, please contact the Technical Support Department at (800) 777-4222 and press 3 (Monday through Friday 8 a.m. – 6 p.m. EST).
For more information: www.fda.gov/MedicalDevices/NewsEvents/News/default.htm

 August 14, 2023
August 14, 2023