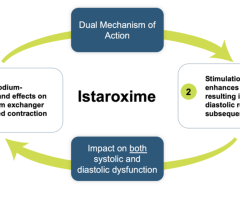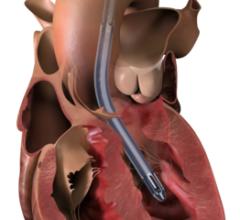
The Impella percutaneous left ventricular assist devices (pLVAD) is an emerging option for partial or total circulatory support. In several randomized, controlled trials, pLVAD therapy demonstrated a better hemodynamic profile compared to IABP, although this did not translate into improved 30-day survival with use of pLVAD therapy.
September 11, 2012 — Primary results from an ongoing trial by Maquet Cardiovascular using intra-aortic balloon pumps (IABP) as an adjuvant treatment for myocardial infarction (MI) complicated by cardiogenic shock have shown no difference in patient survival when compared to standard care alone. Although IABP proved to be safe, the study’s results provide little evidence that use of IABP therapy is associated with hemodynamic improvement, a trait that has been considered critical to its clinical application for more than 40 years. The IABP-SHOCK II trial, conducted between 37 centers in Germany with 600 randomized patients over the course of three years, represents the largest trial ever performed in cardiogenic shock.
Since their introduction in 1968, IABPs have been the most widely used support device in the treatment of MI complicated by cardiogenic shock, with several million people having received treatment using IABP therapy to date. IABP provides temporary, mechanical circulatory support in an attempt to create a more favorable balance of oxygen supply and demand. Through systolic unloading and diastolic augmentation, the cardiac output, ejection fraction (EF) and rate of coronary perfusion are increased, while left ventricular wall stress, systemic resistance to left EF, and pulmonary capillary wedge pressure are decreased. Use of IABP therapy for cardiogenic shock patients during the ‘80s and ‘90s was limited to around 20 percent of cases; however, there has been a temporal trend towards increased use over the years, with rates currently estimated at 25 to 40 percent.
Until now, only a few registry studies and clinical trials have shown that IABP therapy can improve blood pressure and the perfusion of the coronary arteries. Based on this limited data, international guidelines recommend the use of IABP therapy in patients with cardiogenic shock, although, as evident in the frequency of use, many cardiologists are still not entirely convinced of its efficacy. It was for this reason that the IABP-SHOCK II trial was initiated, aiming to provide evidence that use of IABP therapy, currently a Class I recommendation, would reduce the 30-day mortality rate in patients compared to standard care. Besides failing to reduce rates of mortality, use of IABP showed no improvement in blood pressure, no reduction in treatment time in the intensive care unit, no decrease in the duration or dose of drugs prescribed and no improvement in organ perfusion. Several subgroups were additionally evaluated and also showed no clear benefit in using IABP.
While IABP therapy is currently the workhorse circulatory support device used by cardiovascular specialists, GlobalData believes that the Class I recommendation will now be strongly challenged. Previous studies have explored the use of IABP and even reported positive results; however, no trials have had the breadth, statistical significance and randomization of the SHOCK II trial. With this most recent announcement of SHOCK II’s primary results, it is expected that cardiologists will further question continued use of IABP therapy, although six-month and 12-month mortality data must be assessed before any definitive conclusions can be made.
Treatment strategies for patients with cardiogenic shock have come to a key turning point, and results of this most recent IABP trial have left a therapeutic void to fill. Percutaneous left ventricular assist devices (pLVAD) represent an emerging option for partial or total circulatory support and more than one study has compared the safety and efficacy of these devices with IABP. In several randomized, controlled trials, pLVAD therapy demonstrated a better hemodynamic profile compared to IABP, although this did not translate into improved 30-day survival with use of pLVAD therapy. The most common pLVADs studied currently are the TandemHeart developed by CardiacAssist and the Abiomed Impella LP2.5. As pLVAD technology continues to improve, further studies will be required to determine its effectiveness. Muscular counterpulsation exemplifies a noninvasive alternative to IABP therapy and has shown some promise, although more clinical research must be conducted before conclusions can be made.
Besides Maquet, who recently acquired Datascope’s cardiovascular portfolio, other companies with IABP products include Arrow and Abiomed. The results of the SHOCK II study provide a huge opportunity to develop novel and innovative strategies to treat cardiogenic shock, and room for another company to seize market control for this indication. GlobalData expects IABP therapy will be targeted to a more specific target population in future studies where it is deemed most effective, and that pLVAD therapy may undergo further development to improve survival rates.
For more information: www.globaldata.com/ExpertsInsightDetails.aspx?PRID=347&companyID=jpr

 April 28, 2023
April 28, 2023