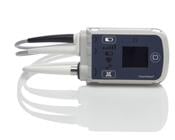
July 19, 2013 — The Mount Sinai Medical Center is the first medical center in New York City to offer the HeartMate II pocket controller, newly approved by the U.S. Food and Drug Administration (FDA), to help its advanced heart failure patients maintain more active lifestyles. This latest-generation controller for the HeartMate II left ventricular assist device (LVAD) is a new small, lightweight, patient-friendly external controller about the size of a smart phone that easily fits in a patient’s front pocket and powers their heart to pump.
“This new HeartMate II controller is a good step forward for our heart failure patients who are awaiting a heart transplant or in need of long-term, permanent support for their survival,” says Sean P. Pinney, M.D., director of the Advanced Heart Failure & Cardiac Transplantation Program at Mount Sinai. “With its smaller size and intuitive safety features, we expect the new controller will enhance the daily lives of our heart failure patients.”
Mount Sinai has one of the largest LVAD programs in the United States, with its cardiothoracic surgeons implanting approximately 50 each year. The center started using LVAD technology in 2006 for advanced heart failure patients. It is used as a bridge-to-transplant (BTT) therapy or destination therapy (DT) for permanent support. In addition, Mount Sinai performs dozens of successful heart transplants each year.
“This new controller will improve the LVAD patient’s overall experience [and] heighten the quality of their life by supporting their active lifestyle, while providing pocket-sized peace of mind for them and their clinical care team,” says Kimberly Ashley, FNP-BC, the VAD program manager for the Advanced Heart Failure & Cardiac Transplantation Program. “We hope to expand the use of this pocket-sized HeartMate II controller to not only new patients but also those current HeartMate II users to give them the option to swap out their old controller for the smaller, safer version, making their lives more comfortable and normal during their daily activities.”
The new patient-friendly controller is a small computer attached to the HeartMate II LVAD via one side cable. It enhances patients’ safety by powering their LVAD to pump their heart, monitoring their vital signs and alerting them of any concerns. Its new design features a diagnostic check system to make sure device wires are intact and functional, special visual alarms, precise onscreen instructions for the patient and longer backup battery power. In addition, the device is programmed with 37 languages to serve diverse patient populations.
“We do extensive patient education training before and after LVAD surgery to teach patients exactly how their controllers work, how to closely monitor their LVAD’s status and also to be vitally aware of any issues or concerns that may arise,” says Bess Griffin, RN, BSN, the VAD coordinator for the Advanced Heart Failure & Cardiac Transplantation Program. “The staff — who have begun training on the new, smart, smaller controller — really enjoy the device’s very easy, user-friendly interface. Thanks to the new controller we are expecting patients may be trained more quickly and feel more confident in caring for their device at home.”
The pocket controller is available for new advanced heart failure patients in need who receive HeartMate II, as well as for current HeartMate II patients who wish to upgrade their existing system controllers.
For more information: www.mountsinai.org


 June 19, 2024
June 19, 2024 









