May 16, 2013 — New research presented at Heart Rhythm 2013 continues to show promising results for focal impulse and rotor modulation (FIRM) mapping to effectively target atrial fibrillation (AF) sources and improves ablation therapy outcomes. The novel diagnostic real-time mapping system helps target ablation therapy to patient-specific drivers of AF rather than to anatomical targets, which can improve patient outcomes.
More than 2 million people in the United States have AF, a common heart rhythm disorder which causes an irregular and rapid heartbeat. Although it is not life threatening, AF can lead to other problems including chronic fatigue, congestive heart failure and stroke.
“This new procedure has the potential to dramatically change how we treat patients with atrial fibrillation,” said Vijay Swarup, M.D., FHRS, director of cardiac electrophysiology at Arizona Heart Hospital. “New findings help us better understand that small electrical spinning tops – rotors – or focal sources drive AF, the stability of these sources and exactly where to treat them.”
New research builds on the CONFIRM trial findings from 2011 that first showed AF was acutely terminated or substantially slowed with less than 10 minutes of FIRM-guided ablation per identified source.
A new study, led by Robert Kowal, M.D., and presented at Heart Rhythm 2013, provides more information on how FIRM-guided ablation effectively treats AF. FIRM mapping was performed in 210 patients at 11 U.S. centers. Ablation was targeted prospectively at rotors or focal sources in 132 consecutive patients, of whom 25 percent had paroxysmal AF and 75 percent had persistent AF. FIRM-guided ablation achieved an acute endpoint in 82.9 percent of patients with ten minutes of total ablation. This included terminating AF and rendering it noninducible in 55.8 percent of patients, in whom the majority (69 percent) terminated to sinus rhythm and 31 percent terminated to atrial tachycardia.
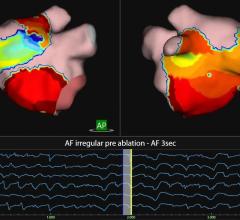
A second study, led by Swarup, used FIRM to map where electrical rotors or focal sources are located in the heart and found that sources exist in diverse stable locations throughout the atria. The 11-multicenter prospective trial mapped AF in 210 consecutive patients, of whom 132 had FIRM-guided ablation at rotor or focal sources of AF. Sources were revealed in 99.2 percent of patients, and more than half of the AF sources were located away from pulmonary veins. These results further confirm the importance of mapping AF sources in each patient compared to traditional pulmonary vein isolation therapy. In FIRM guided patients, AF termination or 10 percent slowing was achieved.
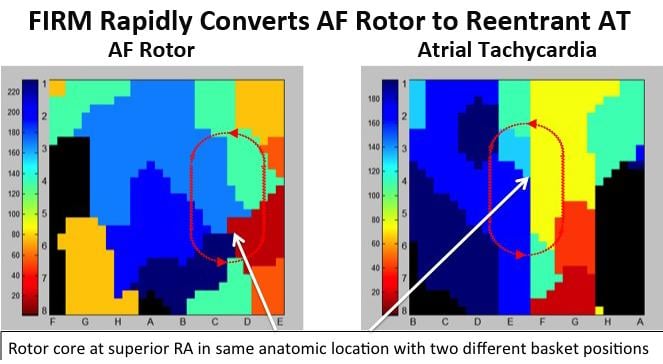
FIRM rotor mapping has spurred some debate in EP because it is believed by some that these rotors move. If they are stationary, the ablations can be very successful, but if they move, as some believe, relief from AF might only be temporary following a FIRM procedure.
For more information: www.HRSonline.org


 September 05, 2025
September 05, 2025