November 20, 2013 — A multidisciplinary team of experts in heart failure, cardiac arrhythmia and neurosurgery at Mount Sinai Hospital in New York City are testing nerve stimulation in the neck as a
therapy for heart failure patients to potentially help relieve their debilitating symptoms of fatigue, shortness of breath and heart arrhythmias while reducing their hospitalizations.
The global, multicenter randomized
clinical trial called "Increase of Vagal Tone in Chronic Heart Failure" (INOVATE-HF) is investigating the safety and efficacy of an implantable vagus nerve electrical stimulation device called CardioFit to improve the heart’s function and the quality of life of heart failure patients.
Mount Sinai’s multidisciplinary team of clinical trial investigators includes principal investigator Vivek Reddy, M.D, electrophysiologist and director of Arrhythmia Services; Brian Kopell, M.D., neurosurgeon and director of the Center for Neuromodulation; and Ajith Nair, M.D., heart failure specialist and director, Pulmonary Hypertension Program, Advanced Heart Failure and Transplantation Program.
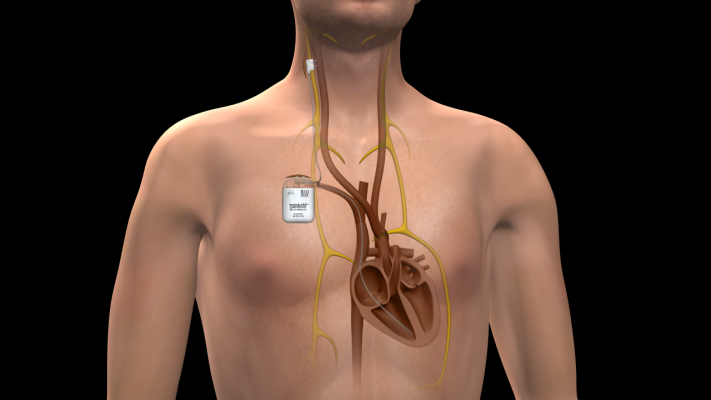
The device is a three-part system consisting of a “stimulator” about the size of a pacemaker and two connecting leads. The stimulator is implanted under the skin of the chest with a “sensor lead” implanted inside the right ventricle of the heart and a “stimulation lead” implanted around the vagus nerve in the right side of the neck.
The large vagus nerve runs from the brain stem down to the abdomen on both the left and right side of the body. It sends signals throughout the body and directly to and from the brain to regulate multiple bodily functions including heart rate.
Once activated by doctors, the stimulator in the chest sends mild electrical pulses up to the vagus nerve to help reduce heart rate, stress and workload on the cardiac muscle to improve overall heart function. The sensor lead in the right ventricle monitors for any abnormal changes in electrical activity and provides feedback to the stimulator enabling it to react.
“This novel use of vagus nerve stimulation may be the therapy we have long been waiting for to bring relief to heart failure patients with chronic symptoms and protect them from dangerous and potentially fatal arrhythmias,” said Reddy. “The results of this study testing the simple electrical stimulation of the body’s powerful vagus nerve may unlock a future promising therapy for heart failure. I am excited that by working with our heart failure and neurosurgery colleagues, we can offer this potentially transformative therapy to our patients.”
“Our clinical trial will compare the benefits of vagus nerve stimulation to the standard treatment of combination medication which is currently our first line of defense against heart failure,” said Nair. “Our study will also test vagus nerve stimulation therapy’s ability to reduce hospitalization, a major issue for heart failure patients, as well as its capability to reduce mortality in this high-risk population.”
“At Mount Sinai, we have successfully used vagus nerve stimulation for refractory epilepsy in patients with uncontrollable seizures and to treat patients with major depression who don’t have good responses to medication therapy,” said Kopell. “This trial represents the first time VNS is being tested in heart failure patients in the United States.”
Recent international studies investigating the CardioFit device have shown that patients with the nerve stimulation device can experience sustained improvement in heart function and structure, heart rate, improvement in quality of life and increased exercise tolerance. Research shows patients may start to experience improvement in their heart failure symptoms after several weeks and months of gradually increased nerve stimulation therapy.
Patients eligible for enrollment in the INOVATE-HF clinical trial are those diagnosed with New York Heart Association (NYHA) stage III “moderate” heart failure who, despite treatment with several combination medications, continue to have debilitating symptoms including fatigue, shortness of breath and abnormal heart arrhythmias.
For more information: www.mountsinai.org