(Editor's note - this article had links to newer content added April 2017)
There have been several advances in electrophysiology (EP) technologies this past year, many of which were highlighted during the Heart Rhythm Society (HRS) 2013 scientific sessions earlier this year. These advances included a leadless pacemaker, new data on contact force sensing ablation catheters, a new electromapping system entering the market and new techniques to map and ablate
atrial fibrillation (AF), said Jagmeet Singh, M.D., Ph.D., director, resynchronization and advanced cardiac therapeutics program at the new Mass General Institute for Heart, Vascular and Stroke Care. Singh also presented numerous sessions at HRS.
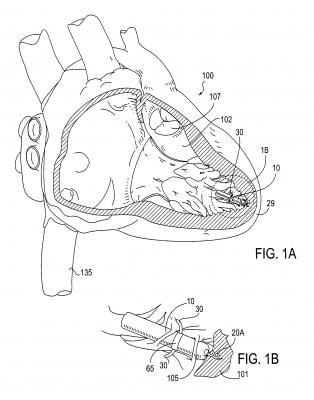
One of the most innovative technologies discussed at the meeting was the Nanostim leadless
pacemaker, Singh explained. The tiny device, about the size of the tip of a pen, is implanted via catheter inside the heart. It attaches to the wall of the left ventricular apex with a screw-in active fixation mechanism. The small size of the device — less than 10 percent the size of a conventional pacemaker — allows for percutaneous placement through an 18 French sheath in the femoral vein, using a steerable catheter.
Nanostim Inc. received CE mark clearance in Europe for the first miniaturized, leadless pacemaker in October. St. Jude Medical, which had in part funded the company’s development efforts, announced immediately afterward it was aquiring the company.
The Nanostim is less than 10 percent the size of a conventional pacemaker. The small size of the device and lack of a surgical pocket, coupled with the exclusion of a lead, improves patient comfort and can reduce complications, including device pocket-related infection and lead failure. The elimination of the visible lump and scar at a conventional pacemaker’s implant site, in addition to the removal of patient activity restrictions that may prevent the dislodgement or damage to a conventional lead, will potentially improve the quality of life for patients.
St. Jude said the Nanostim recently received U.S. Food and Drug Administration (FDA) conditional approval for its investigational device exemption (IDE) application and pivotal clinical trial protocol. The IDE trial will evaluate the Nanostim leadless technology in U.S. patients.
The Nanostim leadless pacemaker was designed to be fully retrievable so that the device can be readily repositioned during the implant procedure and later retrieved if necessary, such as at the time of normal battery replacement.
Even with miniaturization, initial results indicate battery longevity is comparable to conventional pacemakers, with an average lifespan of 8.5 years at 100 percent pacing, according to data from the LEADLESS trial, released at HRS. Data from the 33 patient trial showed a successful implant rate of 97 percent. The mean procedure time was 28 minutes and device performance was evaluated at two days, two weeks, six weeks and three months after surgery. The pacing threshold, R-wave amplitude and impedance were all comparable to that of a conventional pacemaker.
“Leadless pacemakers are a promising new technology that could eliminate one of the biggest complication risks with these life-saving devices — the lead,” said lead author of the study Vivek Reddy, M.D., professor of cardiology, Mount Sinai School of Medicine, and director of the cardiac arrhythmia service at Mount Sinai Hospital, New York City. “Our initial experience indicates that the procedure is faster and minimally invasive compared to traditional implantation surgery, which may dramatically improve recovery times for patients.”
To view a video of how the Nanostim devices is implanted.
Improvements in AF Ablation
There has been increasing interest in new electro-anatomic mapping techniques to visualize the rotation of electrical activation in cardiac tissue that is believed to be the cause of electrical disturbances causing AF. These sites, known as rotors, can be visualized using the
AF FIRM rotor mapping technique, which shows positive data in trials, but also has spurred debate.
“It’s an area of a fair amount of controversy,” Singh said. “There are thoughts that rotors are stationary and other schools of thought that rotors move.”
If they are stationary, the ablations can be very successful, Singh said. However, if rotors move, relief from AF might only be temporary following a FIRM procedure. There are also questions of whether rotors actually drive AF.
The PRECISE study, a late breaking clinical trial presented at HRS, looked at whether FIRM ablation alone can effectively treat AF. The total ablation time averaged about 20 minutes per patient. Patient follow up at nine months showed the success rate was just over 80 percent.
“It was a study with pretty positive results — 82.6 percent is a pretty good response,” Singh said. “This has opened a door into a whole new area of research.”
Another exciting area of discussion in sessions concerned use of noninvasive external beam ablation systems to treat arrhythmias without the need for catheters. Singh said this is possible through advances made with the precision of radiation oncology radiation therapy treatment systems. “It will take a decade or more before it becomes a main stream therapy,” he said.
Electro Mapping Advances
For several years there have only been two
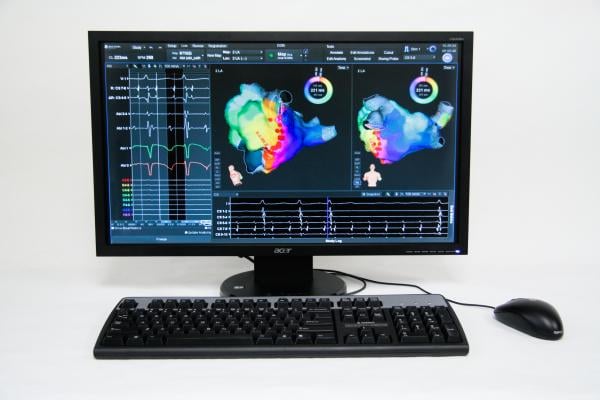
electromapping systems on the market — Biosense Webster’s Carto and St. Jude Medical’s EnSite Velocity — so there is excitement about new players entering the market, Singh said. Boston Scientific's Rhythmia Mapping System, a 3-D mapping and navigation system, garnered a lot of interest at HRS and gained U.S. Food and Drug Administration (FDA) 510(k) clearance this past summer.
Boston Scientific said current mapping systems require a manual, labor-intensive process to create maps, making trade-offs between accuracy and speed. Current systems also offer limited indication of therapy success. The Rhythmia system is designed to intelligently automate map creation, increasing speed and improving the density of mapping. The system also features vMap, a validation map, which is designed to enable electrophysiologists to rapidly confirm the endpoints of the ablation treatment.
"I believe the Rhythmia mapping system will become the new gold standard for mapping and navigation," said Warren Jackman, M.D., professor of medicine, University of Oklahoma Health Sciences Center, when the system gained FDA clearance. "Rhythmia delivers maps of exceptional clarity because it captures thousands versus hundreds of data points. The magic of Rhythmia is continuous mapping. The intelligence built into the system virtually eliminates the need for manual annotation, which is expected to facilitate the diagnosis, treatment and final verification of arrhythmias."
Boston Scientific is offering the Rhythmia mapping system with the company's 64-electrode IntellaMap Orion high-resolution mapping catheter.
Another potential player and possible game-changer is CardioInsight’s noninvasive ECVue 3-D electrocardiographic mapping system. The company said the technology can help diagnose arrhythmias, pre-plan ablation procedures, aid navigation and optimize cardiac resynchronization therapy (CRT) lead implants and pacemaker adjustments. The system uses a 256-lead electrocardiogram (ECG) vest, which the patient wears during a computed tomography (CT) scan. A 3-D reconstruction of the heart is used as a model on which electrical activity is superimposed from the ECG vest. The imaging is created in one heartbeat and produces a colored electromap similar to invasive catheter-based mapping systems. While catheter mapping is time-consuming and only images one chamber, the fast electromapping technique shows the entire heart.
This system already has European market clearance and is FDA 510(k) pending.
“There was a lot of interest in the ECVue system because you can see electrical activation mapping in the ventricles and map the atrium, including rotors. But, this system shows rotors are mobile. So it gives a different prospective to the question of rotors,” said Singh, who serves as the chairman of the CardioInsight advisory board.
Contact Sensing Ablation Catheters
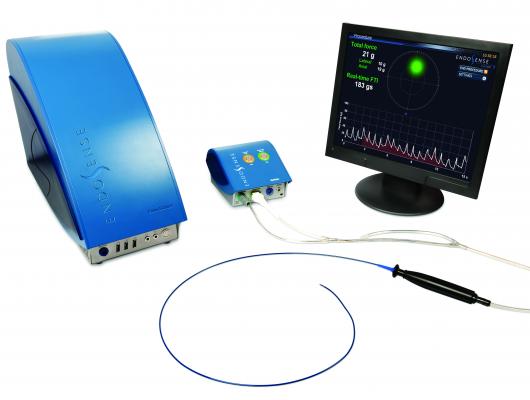
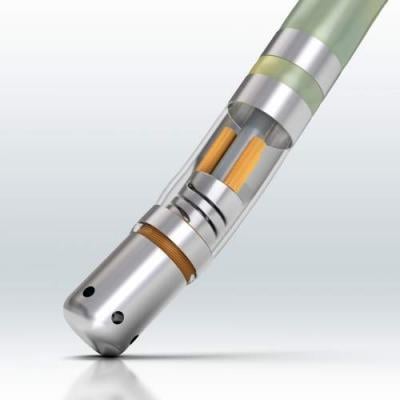
A new trend in transcatheter
ablation technology has been the development of force sensing catheter tips to let operators know if they are applying enough force to guarantee proper lesions to improve patient outcomes. Companies with this technology at HRS this year included Biosense Webster and Endosense.
“There was a fair amount of interest paid to the contact sensing catheters,” Singh said.
At HRS, Biosense Webster announced the 12-month safety and effectiveness results of the Thermocool Smarttouch catheter and software module in the treatment of symptomatic, drug refractory, paroxysmal AF from the SMART-AF investigational device exemption (IDE) trial. The system enables physicians to directly measure contact force, rather than having to rely on surrogate measures.
“SMART-AF showed a 12-month success rate of 72 percent with comparable safety to previous studies,” said data presenter Andrea Natale, M.D., a member of the study advisory committee and executive medical director of the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin. “Furthermore, increased percent of time within physician-targeted contact force range correlated with increased freedom from arrhythmia recurrence, with 84.4 percent of subjects arrhythmia-free at 12 months when the force was within the targeted range >82 percent of the time. This is exciting data for the EP community, which has been working tirelessly to provide better treatments for AF. This will provide an important new tool for treating paroxysmal AF patients.”
The company said the results from the IDE study were submitted to the FDA as part of a premarket approval (PMA) application.
Endosense discussed data from its EFFICAS I prospective multicenter study, which led to the development of guidelines for target and minimum contact force, as well as minimum force time integral (FTI), during the catheter ablation treatment of paroxysmal AF. Published in the April 2013 issue of the American Heart Association journal Circulation: Arrhythmia and Electrophysiology, the newly created guidelines call for a contact force target of 20 grams (and minimum CF of 10 grams) and minimum FTI of 400 grams per individual ablation lesion. The results of EFFICAS I demonstrated the importance of CF in the long-term effectiveness of catheter ablation for treating paroxysmal AF.
While low contact force can lead to ineffective lesions, excessive contact force may cause safety concerns, research has found. In another HRS abstract, “Complementary Techniques and Location of Excessive Contact Force Detection in the Left Atrium with Robotic Catheter Navigation,” Sarah K. Hussain, M.D., of The University of Virginia, Charlottesville, concluded that in a simulated bench experiment, Endosense’s TactiCath ablation catheter could enhance the capabilities of current robotic catheter systems in detecting excessive contact force wherever it occurs in the left atrium.
Advances in Implantable EP Devices
In 2012, Sorin Group demonstrated its SonR technology, which uses a pressure sensor in the tip of the pacing lead to automatically optimize CRT-D therapy. This compensates for cardiac remodeling in heart failure patients. SonR is available in Europe. In March 2013, Sorin gained FDA approval to begin its RESPOND CRT U.S. IDE trial. Singh said the technology detects the first heart sound to gauge contractibility and will automatically adjust the CRT on a weekly basis.
“The SonR system with its innovative sensor is a significant CRT engineering advancement,” said Singh, who serves as the U.S. principal investigator of the RESPOND CRT trial. “RESPOND CRT is the largest trial to date studying this system, and thus we hope to gain valuable evidence further demonstrating that the SonR system offers a significant clinical advancement and can improve heart failure symptoms for a larger number of patients.”
The trial will study the safety and effectiveness of the innovative SonR cardiac resynchronization therapy (CRT) optimization system in patients with advanced heart failure. It is a multicenter, randomized, two-arm, double-blinded, prospective trial that will enroll more than 1,000 patients in the United States and other countries.
At the European Heart Rhythm Association’s Europace Congress in June, Sorin Group announced the results of the DREAM clinical trial, which validated the use of a sleep apnea monitored (SAM) algorithm using Sorin Reply 200 pacemakers. Initial data show that Reply 200 with the new SAM algorithm provides reliable screening for the risk of severe sleep apnea.
Singh said Biotronik introduced two new innovative implantable cardioverter-defibrillator (ICD) systems that only require one lead. In May, the FDA granted market approval to Biotronik’s Ilesto 7 ICD/CRT-D series. The device sends atrial data to ensure diagnostic accuracy and identify previously undetected AF. The second system, the Lumax 740 DX, was cleared by the FDA in February. It is a first-in-class ICD that utilizes a single lead with atrial sensing capabilities. Traditional single-chamber ICDs are designed only to sense changes in ventricular rhythm and are unable to correctly sense atrial arrhythmias, such as AF. This can result in an increased risk of an inappropriate shock or a stroke if AF is not detected.
Improved EP Device Navigation
Medtronic released the CardioGuide Implant System in April. The real-time navigation system for CRT-P and CRT-D devices helps physicians determine the most appropriate location for left-ventricular lead placement using 3-D images of the cardiac veins. Medtronic said an enhanced version of the software, which also analyzes the motion of select cardiac vessels on the left side of the heart, will be available later in 2013.
Paieon Inc. is the developer of the CardioGuide system. Based on its expertise in the field of real-time cardiac fluoroscopy-based image navigation, Paieon has invested considerable engineering efforts in the development of the CardioGuide system. Medtronic has an exclusive licensing agreement with Paieon to market the CardioGuide System worldwide.
St. Jude Medical launched MediGuide in late 2012. It is the first 3-D navigation system intended for the evaluation of vascular and cardiac anatomy on a recorded fluoroscopic image instead of live fluoroscopy. The use of recorded images allows physicians to reduce the duration of radiation exposure during cardiovascular procedures, especially during long EP procedures.
Similar to a global positioning system (GPS) that automobile drivers use to determine the location of their car on a map, MediGuide technology allows physicians to see the precise location and orientation of MediGuid-enabled devices inside the heart. Using magnetic tracking to locate miniature sensors embedded in devices, such as the MediGuide Enabled Livewire Diagnostic Catheter and the CPS Excel MediGuide enabled guidewire, this technology applies 3-D visualization to previously recorded fluoroscopic images in real-time. Automatic adjustments are made to the recorded images to maintain an accurate real-time clinical representation compensating for cardiac motion, respiratory changes and patient movements in order to minimize workflow delays.


 February 06, 2026
February 06, 2026 









