Computed tomography (CT) vendors highlighted several recent improvements to their imaging systems during the 2012 Society of Cardiovascular CT (SCCT) annual scientific meeting in July in Baltimore, Md.
GE highlighted its Discovery CT750 HD FREEdom scanner, which offers a new type of detector for better signal-to-noise ratios and new reconstruction software to reduce stitching artifacts and noise of low-dose scans. It also offers the ability to perform dual energy scans using a single X-ray tube modulated every 0.5 milliseconds between 80 and 140 kVp.
The system’s FREEdom (Fast Registered Energies and ECG) software offers intelligent motion correction via SnapShot Freeze, enhanced coronary visualization even in the presence of calcium, detailed plaque material composition assessment and accurate perfusion calculations. SnapShot Freeze is supposed to significantly reduce coronary motion and overcome the limitation of all hardware-only solutions. It precisely detects vessel motion and velocity and determines actual vessel position and makes corrections.
“SnapShot Freeze will be a game changer by improving the effective temporal resolution, and from the initial images, I see significantly reduced motion artifacts and significantly improved image clarity,” said James Min, director of cardiac imaging research and co-director of cardiac imaging at Cedars Sinai Hospital and current president of SCCT. He is a principal investigator for the VICTORY trial, which is evaluating the diagnostic accuracy of SnapShot Freeze motion and stitching artifact correction.
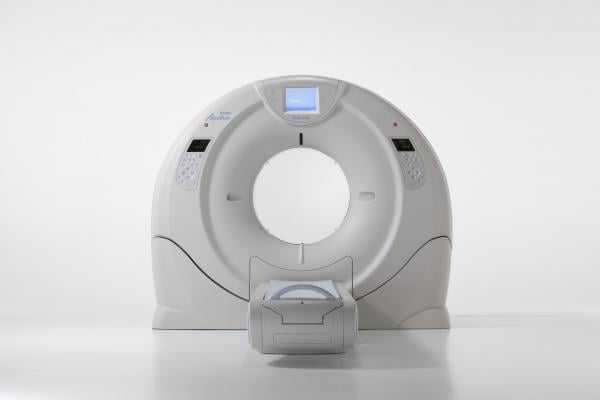
Toshiba’s newest CT system is the Aquilion Prime, which is the replacement for its previous 64-slice system. It is an 80-slice system that can be upgraded in the field over a weekend to a 180-slice system. It offers a 78 mm bore and has 660-pound capacity tables to accommodate larger patients.
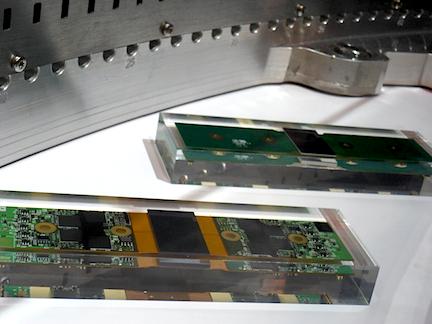
Earlier this year, Siemens introduced the new Stellar detector for its CT systems, which uses integrated, printed microcircuits instead of soldered circuit boards. This reduces the amount of electronic noise in scans by about 20 percent. The new detectors also overall improve image quality by reducing calcium and metal blooming, so it is easier to evaluate in-stent restenosis.


 February 02, 2026
February 02, 2026 









