Transcatheter valve replacement, especially transcatheter aortic valve implantation (TAVI), took center stage at the Transcatheter Cardiovascular Therapeutics (TCT) 2010 symposium this fall in Washington, D.C. This highlights the fact the event has significantly evolved from an angioplasty meeting of 250 interventional cardiologists in the late 1980s. Today the event draws thousands of participants, including cardiac surgeons, vascular surgeons, cardiac imaging and structural heart specialists. These participants underscores a growing trend where the cath lab and its offering of minimally invasive, catheter-based procedures has expanded well beyond coronaries and into nearly all the major vessels of the body. The migration of procedures from operating rooms (ORs) to cath labs or hybrid ORs shows an increased blurring of the lines between specialities that were once silos of expertise that generally did not collaborate well.
PARTNER TAVI Results
One of the most anticipated trials at TCT 2010 was the U.S. PARTNER one-year results. The study compared outcomes of standard therapy and the Edward’s Sapien transcatheter aortic valve in patients who were considered too high-risk for surgical valve replacement. The results showed a major improvement in the transcatheter valve replacement patients. The improvements were so good, it made some experts at TCT question if it will be ethical to randomize patients in the future for standard therapy, which results in generally poor outcomes.
For more details, see the two stories on the PARTNER Trial starting on page 11. Chris S. Malaisrie, M.D., and Attapoom Susupaus, M.D., both from Northwestern University Feinberg School of Medicine, offer an update on the state of transcatheter valve technology.
Transcatheter LAA Occluders
The last issue of DAIC included a story about left atrial appendage (LAA) occlusion devices in development. The devices have the aim of replacing warfarin therapy to prevent stroke in patients with atrial fibriliation. Earlier this year, the FDA reviewed the results of the PROTECT-AF study, comparing the Watchman LAA occluder to warfarin, and decided not to clear the device for use.
Gregg Stone, M.D., New York Presbyterian Hospital, Columbia University Medical Center, speaking at a TCT 2010 session on LAA occluders, said the device shows promise, but failed to allow many patients to discontinue use of warfarin. He said the study also showed the device did not completely seal the LAA. There were also a significant number of adverse events related to implantation, which he said was due to inexperienced operators. “How are you going to become good if you only implant a device every five months?” Stone said. Complications included 30 pericardial effusions, five procedural strokes, four air embolisms, three device embolizations, 16 major bleeding events and 10 cases where surgery was needed to correct these complications.
Saibal Kar, M.D., Cedars-Sinai Medical Center, Los Angeles, said some of these complications were likely due to the Watchman’s sharp, pointed nitinol arms. He said a new device, the Coherex Wavecrest, uses rounded, loop wire arms to avoid perforations. The arms have tiny barbs to more securely anchor the device inside the appendage and avoid embolization.
The Wavecrest is covered in a PTFE (Teflon) membrane, which prevents fluid exchange between the LAA and the left atrium, unlike the net-like material used with the Watchman. This enables ultrasound blood flow imaging to ensure the device is seated properly without any major gaps.
The Wavecrest is currently in pre-clinical studies, but the company is hoping to begin first-in-man trials outside the United States by the end of 2010.
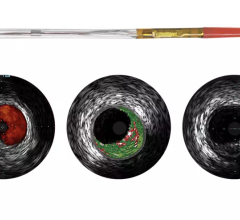
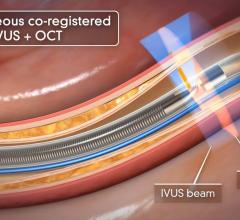
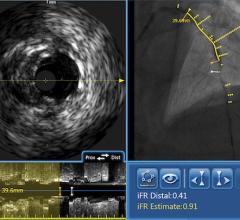
New Intravascular Imaging Modalities
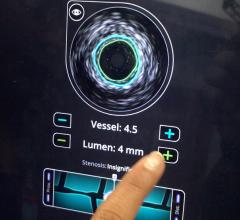
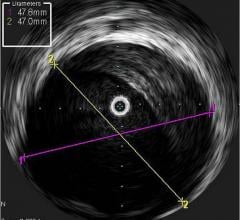
Among the biggest advances on the TCT expo floor were two new FDA-cleared intravascular imaging modalities – optical coherence tomography (OCT) and intravascular ultrasound (IVUS) combined with near-infrared spectrography.
The St. Jude Medical OCT system offers a higher-resolution image than IVUS, allowing clearer evaluation of stent apposition, strut endothelialization and visualization of thrombus.
InfraRedX introduced LipiScan IVUS, which offers grayscale IVUS with a built-in, co-registered spectrograph showing the chemical make-up of plaque. It can help identify vulnerable, lipid-core plaques that are suspected of causing heart attacks and possible adverse events, such as no-flow revascularizations.
For more information on these technologies and advances in intravascular imaging, see the stories starting on page 14.


 September 18, 2025
September 18, 2025