A comparison in size between the current Big Blue Driver unit and the Freedom Driver currently in clinical trials.
The practicality and expense of maintaining patients on total artificial hearts (TAH) may be significantly impacted with the introduction of a new, investigational portable driver unit. The 13-pound unit driver is intended to replace the previous 418-pound driver, which will allow eligible patients to be discharged to wait for a transplant heart at home, rather than in a hospital.
The Challenge
The rapidly increasing burden of patients with advanced heart failure has fueled the growing field of mechanical circulatory support. After remarkable progress over the last several decades, there have been limited recent advances for medical and electrophysiology directed therapies. Heart failure patients are treated under the vigilant guidance of their cardiologists, but their disease inevitably progresses.
Many patients who are candidates for orthotopic heart transplantation, the definitive therapy for late stage cardiomyopathy, die on the waiting list because of limited donor organ availability. Others are referred to transplant centers too late when their heart failure has led to end-organ damage, malnutrition or pulmonary hypertension. Mechanical circulatory support offers these patients hemodynamic stability, improved end-organ perfusion and most importantly, time for recovery of organ function and ultimately heart transplantation.
Circulatory Support Devices
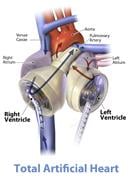
The options for long-term implantable mechanical assist devices for heart failure include left ventricular assist devices (LVAD) and total artificial hearts (TAH). A typical LVAD augments native circulation by drawing blood out of the left ventricle and ejecting it into the aorta, therefore working in parallel to the native heart. A total artificial heart replaces the native ventricles, all four cardiac valves and the proximal portion of the great arteries. By replacing most of the myopathic heart, the total artificial heart eliminates complications including right ventricular failure, arrhythmias, intraventricular thrombus and valvular regurgitation. Candidates for heart transplantation with right ventricular failure, myocardial wall rupture, fulminant cardiac rejection or refractory arrhythmias may benefit from a TAH.
The Artificial Heart
Currently, the only temporary total artificial heart approved by the U.S. Food and Drug Administration (FDA) is the SynCardia temporary Total Artificial Heart (TAH-t). The device consists of two 70 cc pneumatically driven pumps with a total of four tilting disk valves that generate flows of up to 9.5 L/min. The pneumatic cannulae for each ventricle exit from the upper abdomen and connect to the driver. Once the TAH-t is implanted, there are several driver parameters that can be measured and adjusted to optimize perfusion. The device parameters that can be set are: the Total Artificial Heart rate, the left and right pneumatic drive pressures, the percentage of time in systole and the vacuum pressure during filling. The driver monitors both the airflow and driveline pressure, and measures the left and right cardiac outputs. To minimize blood stasis and the risk for thromboembolism, the driver parameters are adjusted such that there is partial filling and complete ejection of blood from both artificial ventricles.
The TAH-t has proven to be effective therapy for bridging severely-ill patients to heart transplantation. There have been over 850 implants and 190 patient years logged on the device worldwide.
Limited Mobility Due to Driver Unit)
Enthusiasm for utilizing the TAH-t, however, has been limited because patients with the device are tethered to a 418-pound pneumatic driver that prevents hospital discharge. While these clinically stable patients continue to rehabilitate in the hospital, they consume valuable space and resources. More concerning is that patients who are on the heart transplant list for a long duration due to blood type or alloimmunity status have a restricted quality of life.
The FDA, however, has recently granted conditional approval for a portable driver for the SynCardia TAH to undergo an investigational device exemption (IDE) clinical study in the United States. Through the IDE study, patients who meet enrollment criteria will have the option to be discharged from the hospital supported by the portable, lithium ion battery power of the Freedom driver. This pneumatic driver weighs 13.5 pounds and is designed to be carried in a backpack or shoulder bag. The device is designed to allow patients with the TAH-t to await heart transplantation at home rather than in the hospital. It is anticipated that this will alleviate the societal and financial burden of extended hospitalization of patients with the older generation driver. While data on the safety and efficacy of this driver will be available only after completion of this study, it has already received CE mark approval in Europe.
While the utility of this therapy must first be proven in the clinical trial, the development of an ambulatory TAH-t driver marks an extraordinary achievement and further advances the field of mechanical circulatory support.
Post Surgical Management
Postoperative management of patients with the TAH-t is similar to the management of patients with LVADs in many respects, although there are some differences. Resuscitation and optimization of end organ perfusion remains essential in the care of these critically ill patients. This is particularly facilitated by the higher pulsatile flow rates possible with a TAH-t and often enables an immediate elimination of the inotropes and vasopressors that are often needed to augment right ventricular function in the use of LVADs. With excision of both native ventricles, complications of arrhythmias and suction events that can occur with LVAD and BiVADs do not occur with the TAH-t.
Patients requiring TAH-t implantation often have significant hepatic congestion related to right ventricular dysfunction and many patients demonstrate a significant coagulopathy in the early postoperative period. Delayed sternal closure is often used in these patients to allow the coagulopathy to reverse and minimize blood loss. A tissue expander is placed in the pericardium to minimize fibrous contraction around the artificial heart and to maintain the space for subsequent transplantation.
Once patients have stabilized following implantation of the TAH-t, they begin an aggressive program of physical rehabilitation. With changes in preload, patients are able to auto increase their cardiac output during exercise. This can be further augmented by increasing the heart rate during therapy as well.
Antiplatelet Therapy
In the TAH-t, the higher flow rates and the short, large diameter path for blood flow lower the thromboembolic risk relative to other devices. Nonetheless, some chronic anticoagulation is necessary, and a combination of aspirin, dipyridamole and warfarin is used. To minimize heparin exposure and the development of heparin induced thrombocytopenia, a direct thrombin inhibitor, bivalirudin, is also used to bridge therapy. A combination of routine coagulation labs as well as the thromboelastogram and platelet aggregation studies is used to titrate therapy.





 January 15, 2026
January 15, 2026 









