March 26, 2010 – When actor John Ritter died suddenly in 2003 from a tear in his thoracic aorta, the tragedy brought attention to a rare but deadly condition that takes the lives of an estimated 10,000 Americans each year. Now, new clinical guidelines spearheaded by the American College of Cardiology (ACC) and the American Heart Association (AHA) not only offer new recommendations for the diagnosis and management of thoracic aortic disease (TAD), they deliver a powerful message to physicians and patients: Early diagnosis and treatment can save lives.
“If thoracic aortic disease can be detected early and managed, it gives us the opportunity to select patients for surgical or endovascular repair when the patient is stable,” said Loren F. Hiratzka, M.D., who chaired the guidelines writing committee and is the medical director of cardiac surgery for TriHealth Inc., Bethesda North and Good Samaritan Hospitals, in Cincinnati. “The results of treatment for stable disease are far better than for acute, and often catastrophic, aortic rupture or dissection.”
The new guidelines appear in the April 6, 2010, issues of the Journal of American College of Cardiology (JACC) and Circulation: Journal of the American Heart Association, as well as on Web sites of the ACC (www.acc.org) and the AHA (www.americanheart.org). They were developed in collaboration with the American Association for Thoracic Surgery (AATS), American College of Radiology (ACR), American Stroke Association (ASA), Society of Cardiovascular Anesthesiologists (SCA), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of Thoracic Surgeons (STS), and Society for Vascular Medicine (SVM). The American College of Emergency Physicians (ACEP) and the American College of Physicians (ACP) were also represented on the writing committee.
Clinical Advances
Recent scientific and clinical advances drove the development of guidelines to aid physicians in the diagnosis and management of aortic dissection, aortic aneurysm and other forms of TAD, said Kim A. Eagle, M.D., director of the University of Michigan Cardiovascular Center in Ann Arbor and co-author of the guidelines.
“We now have a deeper understanding of the genetic underpinnings of TAD, and we continue to expand our knowledge in this area,” he said. “There have been rapid advances in noninvasive imaging. Medical therapy is much better. Open surgical techniques with anesthesia have improved dramatically. We can even use endovascular (minimally invasive, catheter-based) approaches in some patients.”
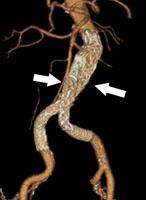
An aortic aneurysm occurs when a portion of the aorta balloons out, increasing the diameter of the blood vessel by at least 50 percent at that spot. Although the wall of the aorta can become dangerously thin, patients with an aortic aneurysm often have no symptoms unless the aneurysm ruptures.
In the case of aortic dissection, a tear in the inner lining (intima) of the aorta allows blood to invade the middle layer (media), creating a false passageway through which blood can flow. This steals a portion of the blood supply from the rest of the body. Classical symptoms include the sudden onset of intense pain in the chest, back, shoulder or abdomen. However, patients often experience less definite symptoms, which makes diagnosis difficult.
In aortic rupture, all three layers of the aortic wall burst, resulting in massive bleeding inside the body.
Risk factors for TAD include poorly controlled high blood pressure, advancing age, male gender, atherosclerosis, inflammatory diseases that damage the blood vessels, and certain genetic conditions that weaken connective tissue, such as Marfan syndrome. In addition, people whose aortic valve has only two leaflets (bicuspid valve) instead of the normal three leaflets may be at increased risk for an aortic aneurysm. Pregnancy, intense weight lifting and cocaine use increase the risk of aortic dissection.
One of the most important messages in the guidelines is that TAD often runs in families. As a result, family history is a critical tool for uncovering undiagnosed cases of TAD. Patients should tell their physicians not only about close relatives with aortic aneurysm, dissection, or rupture, but also about any family history of unexplained sudden death. “Family history is very important,” Dr. Eagle said. “Sudden cardiovascular collapse could have been a heart attack, but it could also have been sudden catastrophic aortic dissection.”
Guideline Highlights
Additional highlights from the TAD guidelines include:
• Imaging of the thoracic aorta by computed tomography (CT), magnetic resonance imaging (MRI) or, in some cases, echocardiography is the best way to detect TAD and determine future risk. A chest X-ray alone is not sufficient.
• Patients with genetic conditions that increase the risk of TAD should have aortic imaging at the time of diagnosis to establish the size of the aorta, with periodic follow-up imaging thereafter.
• All patients with a bicuspid aortic valve should be evaluated to determine whether the aorta is dilating, or widening.
The symptoms of acute aortic dissection, which can mimic those of a heart attack or another cause of chest pain, often make it difficult to arrive at a prompt diagnosis and may delay life-saving treatment. Physicians should keep aortic dissection in mind when asking questions about medical history, family history, and the type and pattern of pain, and when examining the patient.
Aortic dissection involving the ascending aorta (the portion nearest the heart) is a life-threatening emergency that should be treated surgically.
• Aortic dissection involving the descending thoracic aorta may often be managed with medications that control the blood pressure and heart rate, unless life-threatening complications develop. Additional medical therapy may include statins to lower elevated blood cholesterol levels.
• Minimally invasive endovascular techniques are an option in some patients with aneurysm or dissection of the descending thoracic aorta.
• All immediate relatives of a patient with thoracic aortic aneurysm or dissection, or a bicuspid aortic valve, should be evaluated by a cardiovascular physician and undergo aortic imaging to measure the size of the aorta and identify asymptomatic disease.
Dr. Hiratzka said not all health insurers pay for aortic imaging in high-risk asymptomatic patients, particularly based on family history. “I hope the new guidelines will change that,” he said. “It could be lifesaving.”
“People with aortic disease do not have to die prematurely; they can live a long lifespan if they are diagnosed and receive treatment,” said Carolyn Levering, president and chief executive officer of the National Marfan Foundation, which convened the TAD (Thoracic Aortic Disease) Coalition of nonprofit, patient and professional groups. “That’s why the TAD Coalition has come together to launch a comprehensive public and medical awareness campaign to help maximize the impact of the new guidelines. Our first initiative is the dissemination of Ritter Rules, named to honor John Ritter. The purpose of Ritter Rules is to help people remember the important facts about aortic dissection so they can avoid the same kind of tragedy that took the life of the beloved actor.”
For more information: www.acc.org, www.americanheart.org


 February 13, 2026
February 13, 2026 









