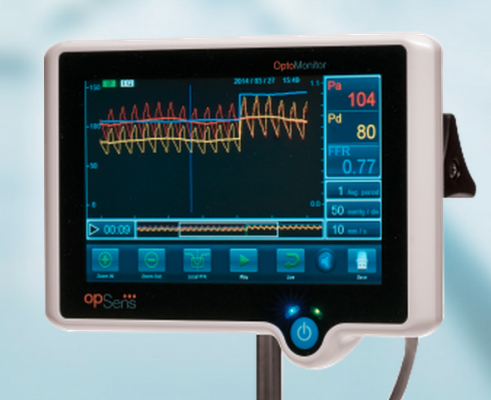
Opsens' new FFR system is the most recent to enter the market, having gained FDA clearance in June.
Key to ongoing U.S. healthcare reform are efforts to reduce costs by eliminating unnecessary medical procedures and using information technology (IT) to justify costs that are incurred to make providers more accountable. Key medical societies in each subspecialty will be responsible for what standards of care should be set and what guidelines should be followed. In the cath lab, fractional flow reserve (FFR) is already viewed as the gold standard for determining the hemodynamic impact of coronary lesions, acting as a gatekeeper to whether a patient is stented or receives medical therapy alone. For this reason, FFR is already being predicted as the measurement of record that will be required in documentation for stent reimbursement in the future.
The proposed Stage 3 Meaningful Use requirements for healthcare IT would require clinical decision support justification for any procedures in the patient's electronic medical record as of 2018, in order receive Medicare reimbursement. This may be one reason why industry sources estimate the FFR market - more than $250 million in U.S. sales in 2013 - is expected to reach $1 billion in the next couple years.
Based on data from FAME and other studies, the Society for Cardiovascular Angiography and Interventions (SCAI) recommends the use of FFR to assess the severity of coronary blockages. It first issued the expert consensus guidance recommendation in November 2013. [2, 3]
FFR determines the functional severity of narrowings in the coronary arteries and pinpoints specifically which lesions are responsible for significantly obstructing the flow of blood, causing ischemia. It is especially useful in patients with several lesions in a vessel segment, of which several might have been stented in the past without use of FFR. The test has shown interventional cardiologists stents are not needed on all lesions that look severe on computed tomography (CT) or angiography, and that lesions that appear mild can sometimes be the culprit of ischemia.
FFR uses a pressure-sensing guide wire fed through a catheter to the site of the blocked artery. The patient is injected with a hyperemic agent (usually adenosine), and the wire measures the flow and pressure of blood before and after the blockage to produce a ratio. Flows of 0.8 and above are deferred for medical therapy, and those below 0.8 are considered flow limiting and stented.
New Players in the Market
Until this past year, the FFR market consisted of only St. Jude Medical and Volcano. However, several new companies recently gained U.S. Food and Drug Administration (FDA) approval for new FFR systems, or are nearing commercialization.
Acist gained FDA clearance for its RXi Rapid Exchange FFR system in January 2014. The device features new technology to provide faster FFR measurements. It uses the ultra-thin Acist Navvus Rapid Exchange MicroCatheter and RXi console. The catheter can be used over a standard 0.014-inch guide wire, providing the physician maximum control while maintaining wire position throughout the coronary procedure. In addition to using a rapid exchange catheter, the Acist system utilizes fiber-optic technology for greater signal stability and less potential for signal drift. The company said its Navvus MicroCatheter offers simple plug-and-play usability without the need to calibrate it, saving time and increasing ease of use versus older FFR wire-base systems.
In August 2014, Acist entered into a strategic agreement with Medtronic to co-promote a combined RXi FFR system with high definition IVUS (HDi) technologies in the United States.
Boston Scientific launched its new Polaris intravascular ultrasound (IVUS) imaging system that is FFR-capable in July 2014. It also announced a partnership a month later with Asahi Intecc to develop a new, differentiated FFR wire. The joint project focuses on creating a device intended to improve handling compared to existing FFR wires. Boston Scientific anticipated commercializing its FFR wire in 2015.
Opsens Inc. is the most recent entry, gaining FDA clearance in June for its OptoWire and OptoMonitor FFR system. Its first commercial systems entered service this past January in Europe. The OptoWire provides intra-coronary blood pressure measurements with optical pressure guide wire technologies. The company said it is immune to adverse effects related to blood contact and allows easy connectivity that leads to reliable FFR measurements in extended conditions of usage.
Philips Healthcare purchased Volcano last December for $1 billion to add its FFR and IVUS technology to its interventional lab portfolio.
Adenosine-free FFR Introduced
Volcano Corp. gained FDA clearance for its instant wave-free ratio (iFR) modality in April 2014. iFR is a physiologic measurement performed using the same pressure wires and equipment utilized in FFR, but does not require the injection of hyperemic agents into the patient that induce stress to the heart. Some interventional cardiologists have hesitated using FFR because adenosine may not be well tolerated in some patients and adds time to the procedure.
iFR assesses the pressure difference across a stenosis during the rest period of the cardiac cycle and allows for a lesion-specific assessment in seconds by amplifying the resting pressure waveform. It is used most efficiently with Volcano's Verrata Pressure Guide Wire, which is designed for simple disconnection and reattachment during a procedure and facilitates making quick measurements multiple times during a procedure without injecting hyperemic agents each time.
The VERIFY study[4] compared iFR to FFR for diagnostic accuracy, which found iFR correlates weakly with FFR. In the trial, compared to the FFR cut-off value of ≤0.80, the diagnostic accuracy of the iFR value of ≤0.80 was 60 percent for all vessels studied. It was 51 percent for those patients with FFR in the range of 0.60 to 0.90.The study also found iFR was significantly influenced by the induction of hyperemia.
For this reason, operators using iFR often have a gray-area cut-off range where iFR is uncertain and they will switch over to FFR with induced hyperemia. Using this model, operators say they can still greatly reduce the number of patients requiring FFR. For iFR measurements greater than 0.93, stenting is not necessary. Measurements less than 0.86 are treated by revascularization. If iFR measurements fall within the gray zone (between 0.86 and 0.93), an FFR assessment is performed. Using these benchmarks, at least 60 percent of patients are spared from vasodilator infusion.
In April, Philips announced European CE mark clearance for the Volcano iFR Scout pullback software. This is a functional extension of the existing Volcano iFR modality, optimized to assess serial lesions and diffuse coronary disease. Physicians have historically used a pressure wire pullback technique with FFR, under hyperemic conditions, to assess the type of underlying disease severity, whether focal or diffuse, to help determine the appropriate treatment for the patient. With the iFR Scout pullback software, this same functionality to scout out the most treatable lesions is now available without the administration of a hyperemic agent, thereby potentially reducing procedural time and cost to the facility, as well as improving patient comfort. This software is currently pending 510(k) clearance with the FDA.
Read the article "FFR and iFR in the Diagnosis and Treatment of Heart Disease"
Watch the VIDEO "iFR Equal to FFR Outcomes in Coronary Lesion Evaluation."
FFR Systems Comparison Chart
This article served as an introduction for charts on FFR catheters and systems. The FFR Systems Comparison Chart can be accessed at www.dicardiology.com/content/ffr-systems. The FFR Catheters Comparison Chart chart at www.dicardiology.com/content/ffr-catheters.Access to the charts requires a login, but it is free and only takes a minute to complete.
References:


 November 14, 2025
November 14, 2025 









