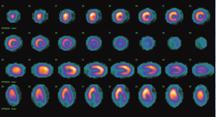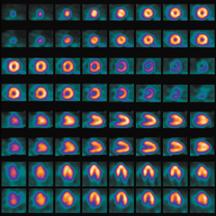
Lantheus Medical Imagings SPECT radiotracer Cardiolite is the most widely used technetium-99 agent for myocardial perfusion studies.
In what some might argue is similar to the industry shake-out between Blu-ray and HD digital video disc technology, the competition between SPECT and PET cardiac imaging is heating up. And the marketing of existing radiotracers and the development of new nuclear imaging agents is reflective of both advances in technology and clinical trends.
While PET offers high resolution images and a 75-second half-life for fast imaging, SPECT is cheaper, more commonly used and allows imaging over several hours. Advancements in radiotracers will boost the capabilities of both modalities, adding more factors into the debate over which is better.
Advances in SPECT
GE Healthcare remains a major player in the SPECT market with its radiotracer Myoview (technetium Tc-99m tetrofosmin), which is effective in the diagnosis and localization of regions of reversible myocardial ischemia in the presence or absence of infarction under both rest and stress. Nathan Hermony, GE Healthcare’s global business leader for nuclear medicine, said Myoview has a half-life of six hours, so it can be shipped as a unit dose as needed via next-day delivery.
While Hermony admits PET image resolution is superior to SPECT, he said the use of PET radiotracers requires an in-house generator, or for N-13 Ammonia access to a cyclotron, because these agents must be prepared just before injection. That adds to the cost of PET. “If you’re a small clinic, it’s just not profitable,” he said.
Hermony said GE Healthcare introduced a new system for SPECT scanners using tiny detectors “to acquire data from all angles,” which will better show blood flow into the myocardium. “We believe this new technology will improve SPECT,” Hermony said. Announced March 25, Alcyone technology is a nuclear cardiology platform combining cadmium zinc telluride detectors, focused pin-hole collimation, 3D reconstruction and stationary data acquisition. The new technology will be available on both the Discovery NM 530c and the Discovery NM/CT 570c. Alcyone’s heightened sensitivity and zero equipment motion will improve both image quality and energy resolution, enabling the potential for new clinical applications including 3D dynamic acquisitions and simultaneous dual isotope imaging, Hermony said.
In addition, he said the company recently developed a new radiotracer for use in diagnosing heart failure. Not yet cleared by the FDA, it is currently being marketed in Europe. Called AdreView (Iobenguane I 123 Injection), the product is a molecular imaging agent that has the ability to assess the nerves of the heart on the cellular level, which cannot be done using standard tests such as echocardiograms.
Preliminary results were presented at the American College of Cardiology’s 58th Annual Scientific Session in Orlando, FL on March 31. Arnold F. Jacobson, M.D., Ph.D., head of GE Healthcare’s Cardiac Center of Excellence and lead investigator of the study entitled “The Prognostic Significance of 123I-mIBG Myocardial Scintigraphy in Heart Failure Patients: Results from the Prospective Multicenter International ADMIRE-HF Trial,” said this new agent will give clinicians the ability to view the heart’s function at the molecular level. “The ADMIRE-HF study data suggest that use of this imaging test may provide a better understanding of a patient’s heart function and the risk that it may worsen over time, which is particularly important for heart failure patients,” Dr. Jacobson said. “This new information, in conjunction with currently available tests, may help clinicians better understand the severity of their patient’s condition and thereby aid in their management decisions.”
Another company that continues to make strides in the SPECT field is Lantheus Medical Imaging. Its Cardiolite (technetium Tc99m Sestamibi) is the most widely used technetium-99 agent for myocardial perfusion studies, says Mark Hibberd, M.D., Ph.D., senior medical director, Global Medical Affairs. “It’s past its patent life, but it’s still the most widely used agent. It has been used on more than 40 million patients worldwide,” he said, adding that Cardiolite has captured about 60 percent of market share. On the market for about 14 years, Cardiolite has a half-life of six hours and costs, on average, $40 to $50 per dose.
“You can give the agent and image later, within a few hours, and still make an accurate diagnosis,” Dr. Hibberd said.
Unlike PET agents, whose short half-life typically rules out treadmill-induced stress tests, SPECT agents like Cardiolite allow for such tests. “Cardiologists prefer exercise stress rather than pharmaceutical-induced stress,” Dr. Hibberd points out.
A company that is making in-roads into both PET and SPECT is Covidien Medical Imaging. In September 2008, the FDA granted approval for Covidien’s generic kit for the preparation of technetium Tc99m Sestamibi for myocardial perfusion imaging. This product now is the generic version of Lantheus Medical’s Cardiolite. The generic product is available in the U.S. as well as in Denmark, Germany and the U.K.
In January, Covidien and Babcock & Wilcox Technical Services Group agreed to jointly develop technology to produce Mo-99, the parent isotope of technetium-99. Under the agreement, B&W TSG and Mallinckrodt, a subsidiary of Covidien, will work together on an aqueous homogeneous reactor design originally developed by B&W in the 1950s.
Also called a solution reactor, the configuration potentially bypasses many pitfalls encountered with conventional fission reactors during the production of Mo-99. One of the biggest issues include shortages of imaging isotopes when current reactors are shut down for any reason.
The B&W TSG-Mallinckrodt collaboration has the potential to supply more than 50 percent of U.S. demand, says Jud Simmons, public relations manager for B&W. No site for the reactor and processing plant has been chosen, he said, and it will probably take five to six years before production of Mo-99 can begin, he noted.
Molecular Insight Pharmaceuticals is developing a targeted SPECT tracer for cardiology. The company’s lead product candidate is Zemiva, iodofiltic acid I 123, a fatty acid analog that detects cardiac ischemia by revealing abnormalities in fatty acid metabolism in the heart. Normally functioning cardiac muscle primarily uses fatty acids as its energy source. Under ischemic conditions, heart tissue converts to carbohydrate metabolism. The drug exploits this change. The company said normal heart tissue absorbs Zemiva, but ischemic tissue does not.
Most perfusion studies must be performed within two hours after the cessation of heart attack symptoms, but the company said Zemiva can identify sites of ischemia for up to 30 hours after symptom cessation.
Recently released data from a Phase 2 trial of 510 emergency department chest pain patients showed Zemiva significantly increased sensitivity from 52.2 to 85 percent and negative predictive value from 72 to 89.6 percent. The company is currently planning its Phase 3 trial, which is scheduled to begin by early 2010.
PET Agents Gaining Ground
Bracco Diagnostics still appears to dominate the PET tracer market with its flagship product, CardioGen-82. “CardioGen has been out since 1989 and has shown significant growth year over year,” said Kim Giordano, the company’s vice president of nuclear medicine. “About 93 percent of studies done are with CardioGen.”
In addition, she noted, “CardioGen-82 is the only FDA approved generator-base PET perfusion agent for CAD (coronary artery disease).”
Bracco has an agreement with Lantheus under which Lantheus can sell CardioGen. “We wanted to partner with a company that could get more feet on the street,” Giordano said. “The contract will always be a Bracco contract, but they are promoting it. They’re acting as an agent.”
To expand its cardiovascular offerings, Lantheus is developing PET radiotracers. Phase II clinical trials have begun for BMS747158, a fluorine-18 labeled agent that binds to the mitochondrial complex one inhibitor and was designed as a novel myocardial perfusion PET imaging agent.
Thus far, the agent has exhibited high target to non-target uptake ratios, perfusion defect recognition and very high image quality. “It will show which vessels require intervention,” explained Dr. Hibberd. “It will reduce false positives in myocardial perfusion imaging studies because it eliminates artifacts in the image.”
He said this agent should be introduced in 2011 or 2012.
The company also has a congestive heart failure PET agent in development, he said. Clinical trials for the heart failure agent should begin later this year.
As PET imaging gains wider acceptance, other companies are ratcheting up their R&D cycles.
FluoroPharma Inc., which is now part of QuantRx Biomedical Corp., has two agents in clinical trials, says David Elmaleh, M.D., associate professor of radiology at Harvard Medical School, director of contrast media at Massachusetts General Hospital and FluoroPharma’s chief scientific advisor. “One looks at blood flow to the heart and the other looks at the integrity of the heart,” he said.
In March 2008, FluoroPharma announced positive Phase I safety results for CardioPET, the company’s lead product, which is designed for myocardial imaging of healthy patients and those with CAD.
Using a fatty acid analog, CardioPET allows assessment of acute and chronic CAD while patients are at rest, thereby eliminating the need for standard stress testing.
Phase I trials also were completed last year for BFPET, a novel fluorine-18 labeled tracer for myocardial infusion imaging that has a half-life of about two hours. Preliminary tests showed fast blood clearance, rapid and stable myocardial uptake and high heart to background ratios.
Dr. Elmaleh said FluoroPharma is seeking funding to begin tests on another agent, called VasoPET, which would be used to assess the formation of atherosclerotic plaque. In January, this F18 labeled 2-fluorodeoxy-D-glucose (FDG) tracer was issued a patent, exclusively licensed from Massachusetts General Hospital.
On March 20, PETNET Solutions, a wholly owned subsidiary of Siemens Medical Solutions and Covidien announced the opening of a new SPECT radiopharmacy in Raleigh, NC. The new site is located next to PETNET’s existing PET manufacturing and distribution center. It is USP Chapter 797-compliant and is the only site in central and eastern North Carolina with a cyclotron and the ability to service both high and low energy isotopes.
Room to Grow
As to whether PET will eventually overtake SPECT for the majority of cardiac imaging studies, Bracco’s Giordano said, “The wave of SPECT imaging is declining.”
“The number of PET centers are expanding rapidly,” added Dr. Hibberd. “The technology for PET imaging will improve over time. Equipment will evolve and will become more readily available and less expensive. And the cost effectiveness of PET agents will become evident.”
For More Information
www.bracco.com
www.covidien.com
www.fluoropharma.com
www.gehealthcare.com
www.radiopharm.com