Until recently, cardiologists trying to diagnose and treat arrhythmias have had to deal with technological limitations in data transmission, storage and analysis. We have had to rely on third-party independent diagnostic and testing facilities (IDTFs) to read remote cardiac monitoring data and then phone, fax or e-mail the important events back to the practice.
The Blind Spots in Remote Cardiac Monitoring

A big problem is that 95 percent or more of the data gets lost in transmission. IDTFs may catch an arrhythmia during the monitoring period, and send the physician a short 20- or 30-second strip. But too often, the cardiologist never sees the critical onset and offset data in the minutes immediately prior to and after the arrhythmia.
Legacy ambulatory monitoring companies only provide patient-triggered or algorithm-triggered events for evaluation by the physician. This accounts for far less than 5 percent of the recorded data. Physicians have no easy way to verify the accuracy of the data or gauge what might be missing.
It is like looking at the sky through the wrong end of the telescope. The data loss can be immense. Many vendors claimed to offer full disclosure, but still only transmitted a few glimpses, measurable in seconds, of the patient’s cardiac data to the doctor. Verification can be difficult, or in some cases impossible, and if a doctor has questions, they need to spend time calling the IDTF call center, get transferred around and explain their question and wait for the IDTF technician to access any data they have.
The process is slow and expensive, and results in massive blind spots for the care provider trying to diagnose and treat the cardiac patient.
Eliminating Remote ECG Detection Delays
Even when an IDTF captures critical data, the process of actually getting the information in front of the physician for diagnosis and intervention can take hours and even days. In practice, the patient could potentially have a rhythm abnormality on a Holter that the physician and patient are not aware of for another week.
More than half of all clinically significant arrhythmias are reported after a typical two-day Holter monitoring period is over. Devices that rely on self-activation usually miss the critical first few minutes of onset data. Additionally, one in four patients is unable to activate the device during a cardiac event.
If an undiscovered dangerous arrhythmia is detected, this inefficient process leaves the patient vulnerable. Eliminating these delays can avert syncopal episodes and potentially sudden cardiac death (SCD).
New technology in the field today can help streamline the process, and quickly eliminate the need for these costly IDTF intermediaries.
Recent advances in HIPAA-compliant telecommunication and data analytics now enable secure transmission of full-disclosure, telemetry-like data throughout the monitoring period, in near real-time, directly to the physician’s tablet or smartphone. Exciting new technological developments are bringing rapid improvements to remote cardiac monitoring. These include next-generation remote cardiac monitoring devices that use deep-learning and software as a service (SaaS) remote cardiac monitoring platforms. These newer monitors and accompanying software can help realize the elusive promise of remote cardiac monitoring platforms with:
• Systems that truly replicate in-hospital monitoring;
• Full-disclosure cardiac data;
• Direct delivery to physicians — bypassing the IDTF;
• 24/7, on-demand review.
One example of this trend is InfoBionic’s MoMe Kardia technology. It allows the doctor to set customizable alerts and makes it easy for them to quickly scroll through the minutes and hours before or after a known cardiac event to quickly identify critical onset and offset data. This data is routinely missed by legacy monitoring systems, since it is not included in the 20-30 second strips the physician receives from the IDTF.
The newly-emerging ability to transmit full-disclosure data throughout an extended monitoring period directly to the physician’s mobile device, and enable early access to the data during the monitoring period, is game-changing and promises to disrupt the cardiac monitoring industry.
Superior Diagnostic Yield With New Generation Remote ECG Monitors
First, the clinical benefits are tremendous. These full-disclosure devices extend the monitoring period up to 30 days — with the physician having full, unfettered access to the complete heartbeat-to-heartbeat data the entire time. Studies have shown that the longer the monitoring, the more likely it is to pick up either a symptomatic or asymptomatic arrhythmia — and there is a better chance at correlating symptoms or activities with cardiac events.
Improved Verification and Diagnosis Using Remote Cardiac Monitoring
With extended full disclosure with real-time monitoring for up to 30 days per fitting, the technology now allows doctors to directly verify compliance, atrial fibrillation (Afib) burden and premature ventricular contraction (PVC) burden.
Subscription-based SaaS software platforms also allow doctors to go directly to the online electrocardiographic data, from any computer anywhere in the world. They can scroll backward and forward to see when the event started, how much arrhythmia is there, how well the patient complied with the monitoring.
Better Responsiveness to Cardiac Events
The new generation of remote cardiac monitors also allow physicians easy access the data from any computer or smartphone at any time after an alert. If a patient calls experiencing symptoms, or if the cardiologist receives an automated alert on their mobile device, the doctor can see the patient’s nearly up-to-the-minute data on the spot and make a critical cardiac arrhythmia diagnosis
The after-hour notification feature is particularly beneficial, allowing the doctor immediate access to the patient without the need for contacting a call.
Improved Patient Compliance

The new generation of cardiac monitors also enable improved compliance over the IDTF model of mailing a device to patients with instructions for use. Today, the doctor’s office staff can fit and train the patient on the wear and use of the monitor in person.
Newer monitors are also much more lightweight, patient-friendly and simpler to use. Some of these devices have a single button for patients to press and do not require an external cell phone or “brick” to carry around. This can significantly improve patient compliance. This also is important when a typical patient who is 75-years old may struggle with using new technology.
The photo shown here is of the Cardea Solo wearable ECG monitor, which is an example of the newer generation wearable monitors that are replacing traditional Holters.
A New Business Model for Cardiologists
Today’s monitors and the business model provided by digital health companies also allow physicians’ practices to open new revenue streams. Because the cardiologist or primary care physician is getting a direct full-disclosure feed throughout the monitoring period, they can also bypass the IDTFs and bill globally for both the clinical and the technical monitoring service. This is of vital interest not only to doctors, but to hospital administrators and practice managers, because it potentially substantially increases the provider’s revenue per patient.
Editor’s note: Stuart Long is the CEO of InfoBionic, the developers of the MoMe Kardia remote cardiac monitor. Colin Movsowitz, M.D., is a Philadelphia-based cardiologist who uses the MoMe Kardia 3-in-1 monitor in his practice.
Reference:
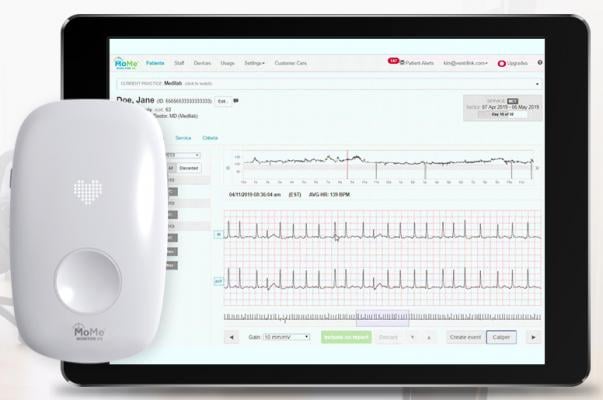
 A big problem is that 95 percent or more of the data gets lost in transmission. IDTFs may catch an arrhythmia during the monitoring period, and send the physician a short 20- or 30-second strip. But too often, the cardiologist never sees the critical onset and offset data in the minutes immediately prior to and after the arrhythmia.
A big problem is that 95 percent or more of the data gets lost in transmission. IDTFs may catch an arrhythmia during the monitoring period, and send the physician a short 20- or 30-second strip. But too often, the cardiologist never sees the critical onset and offset data in the minutes immediately prior to and after the arrhythmia.  The new generation of cardiac monitors also enable improved compliance over the IDTF model of mailing a device to patients with instructions for use. Today, the doctor’s office staff can fit and train the patient on the wear and use of the monitor in person.
The new generation of cardiac monitors also enable improved compliance over the IDTF model of mailing a device to patients with instructions for use. Today, the doctor’s office staff can fit and train the patient on the wear and use of the monitor in person. 


 December 19, 2025
December 19, 2025 









