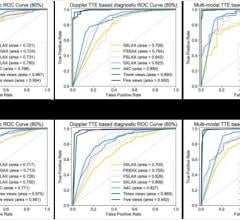
July 3, 2014 — More than 55 companies displayed their latest products and services at the American Society of Echocardiography (ASE) 25th Annual Scientific Sessions June 20-24 in Portland, Ore. The meeting is the largest for cardiovascular ultrasound. Highlights from the show floor included:
The ASE Foundation demonstrated Version 2.0 of its free mobile Echo Apropriate Use Criteria (AUC) app on iPads. The app, made possible by a grant from the ABIM Foundation and supported by the Robert Wood Johnson Foundation, was designed to guide physicians when ordering testing in cardiovascular care and echocardiography. The latest update includes three new echo modalities, six new stress echo algorithms and additional Choosing Wisely "Lists of Things Physicians and Patients Should Question" lists.
Axess Ultrasound showcased the latest testing methods used in order to properly diagnose transespohageal probes. Newest TEE repairs include total repair capabilities on the Acuson V5Ms along with minor repairs for the GE 6T/6Tc and coming soon, the Philips X7-2t.
Biodex Medical Systems redesigned its imaging tables for echocardiography, with ergonomic enhancements for improved imaging. The new cardiac drop-down cushion releases and returns from either side of the table, providing open access to the patient’s left thorax area for an unencumbered apical approach. A motorized Fowler back, infinitely adjustable 0-80 degrees, accommodates natural body extension and sitting position without slide or shift. Redesigned flush-mounted side rails enable closer access to the patient without sonographer contortion. When not in use, rails are stowed beneath the table, eliminating obstruction.
The British Society of Echocardiography showcased two new 2014 ventures. Inspired by the ASE's program in India, the BSE will undertake its first humanitarian project in South Africa where volunteers will screen up to 2,000 school students from low socio-economic communities in and around Cape Town for early stage rheumatic heart disease. This five year scanning and research initiative is in collaboration with Stellenbosch University, Tygerberg Hospital.
CAE Healthcare demonstrated Vimedix, the only ultrasound simulator with the transthoracic, transesophageal and abdominal-pelvic exams on one platform.
Digisonics showcased a new feature for its cardiovascular information system solutions called Smart, Structured Macros and Report Templates. Developed for complex pediatric reporting workflows and other intricate clinical specialties, Smart provides users with the convenience of quickly creating an entire report template through an intuitive, structured workflow. Two new products also being exhibited include DigiConnect, which launches third-party systems such as EMR/HIS or ECG management software directly from the Digisonics workstation with a single sign-on, and WebView 4.0 for zero-footprint image and report review from any HTML5 capable browser or operating system.
Epsilon Imaging showed a vendor-neutral suite of software applications designed for echocardiography. EchoInsight provides a suite of clinical applications for visualization and analysis using practical strain imaging. Applications assist clinicians to enhance, standardize and streamline interpretation and reporting of echo studies. Initial applications include cardio oncology, RV and stress echo. Features include an automated measurement suite based on ASE guidelines, practical application-specific strain imaging, and rapid study comparison and trending for comprehensive assessment and patient monitoring in echo.
Esaote North America highlighted its new CrystaLine imaging technology that enhances image clarity and provides the ability to personalize image quality for each patient. MyLab ultrasound systems with CrystaLine technology allow sonographers to "dial in" the right amount of speckle for each patient to increase image quality across diverse patient populations. Images for all patients now yield better anatomical definition throughout extended fields of view, leading to a more confident interpretation of image data.
Fujifilm demonstrated its latest generation of Synapse Cardiovascular, V5.1, which allows cardiologists and clinical professionals to enhance patient care through advanced reporting applications. At the show, customers were able to see a works-in-progress demonstration of Synapse Cardiovascular 6.0, which will introduce new functionality for echocardiography. Fujifilm also demonstrated its zero-footprint mobile application, Synapse Mobility. The application enables access to Fujifilm’s suite of Synapse products from hand-held devices, in addition to browser independence.
GE Healthcare introduced its new cardiac and shared services ultrasound system called the Vivid T8. It combines the established cardiac imaging capabilities of GE Vivid systems with exceptional shared services performance of the company's Logiq systems. The result is a truly hybrid cardiovascular ultrasound system that is rugged, reliable, robust and rich with features, yet still affordable and convenient to use. It comes with three years of comprehensive service coverage.
Hitachi Aloka Medical introduced its new Arietta cardiovascular ultrasound platform. The vendor also unveiled new software that enables vector flow imaging and analysis.
Medical Positioning Inc. demonstrated the latest versions of its EchoBed and EchoTable, which are designed to improve diagnostic imaging and procedures, as well as maximize sonographer ergonomics. MPI’s products significantly improve the images, leading to better diagnoses and patient outcomes with ease of use for both sonographers and patients.
Medstreaming showcased the latest version of its Cardiovascular Datacenter, which establishes an exchange for the consolidation and dissemination of clinical-based evidence for multiple modalities supporting all standards-based vendors’ equipment. Medstreaming Cardiovascular Datacenter affords both reading and referring physicians the capability to monitor and review a patient’s images and reports.
Philips Healthcare showed its Anatomical Intelligence software, available on its new Epiq premium echocardiography system, which delivers new levels of clinical information, advanced organ modeling and quantification to make exams easier to perform and reproduce.
Siemens demonstrated release 3.5 of the Acuson SC2000 volume imaging ultrasound system, a fully functional 2-D and volume imaging ultrasound system that expands the usability of Siemens’ established SC2000 system further into abdominal vascular applications. Also showcased was the Acuson X700 ultrasound system, which offers exceptional image quality and intelligent workflow solutions at an excellent price/performance ratio, and the Acuson P300 ultrasound system, which now features a stress echo package. The Acuson Freestyle system, the first ultrasound system featuring wireless transducers, offers improved procedural productivity and enhanced workflow efficiency.
Simbionix featured the latest updates to its Ultrasound Mentor platform, which offers highly realistic transespohageal echocardiography hands-on training within a standardized, educationally enhanced environment. Combining true-to-life patient scenarios at adjustable severity levels with a comprehensive clinical setting, the simulation provides training to the full extent of the actual procedure. Basic to advanced tools including ultrasound image controls, color and spectral Doppler, M-mode, cine loops and more are at the user’s disposal. Clinical findings documentation and captured performance metrics support self-assessment, as well as monitored progress towards proficiency prior to ever performing on a real patient.
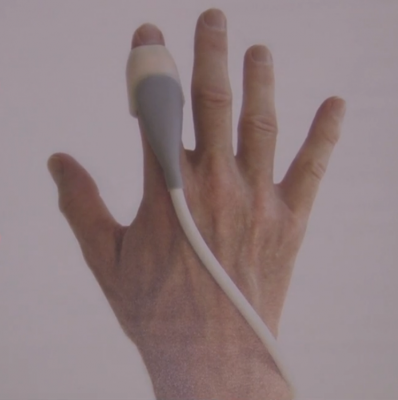
Sonivate Medical demonstrated its revolutionary SonicEye, a finger-worn ultrasound probe. The SonicEye is an intuitive device that allows clinicians to simultaneously “see, feel, and do” in a variety of medical applications. Because it is one of the first medical devices that is truly wearable, the SonicEye finger probe can be used with ultrasound systems and other portable devices, such as Google Glass, to create a total wearable experience.
TomTec Imaging Systems showcased the latest versions of its Image-Com and Echo-Com software solutions for multimodality imaging and echocardiography. Image-Com has been developed to speed up workflow in the daily routine, which makes cardiological information available faster, provides additional clinical information and accelerates reporting.
Toshiba America Medical Systems showcased its Aplio 500 CV, 300 CV and Artida with wall motion tracking to help identify early stages of cardiovascular disease. From unsurpassed image quality that delivers exceptional depth and detail, to sophisticated ergonomics and software enhancements that elevate efficiency to unmatched levels, these systems define a new era in performance.
Ventripoint Inc. showed its VentriPoint Medical System (VMS), which received U.S. Food and Drug Administration (FDA) clearance for use in adults with pulmonary hypertension. The technology has been determined substantially equivalent to magnetic resonance imaging (MRI). The VMS allows cardiologists to obtain a 3-D volume rendering of the right ventricle using 2-D ultrasound. The result is automatic quantification of end-diastolic volumes, end-systolic volumes, ejection fraction and shape. Multiple peer reviewed studies have shown VMS to be useful in pediatric and adult patients for a variety of cardiac conditions, including pulmonary arterial hypertension, tetrology of Fallot and systemic right ventricles. Along with FDA clearance, VMS also maintains CE and Health Canada regulatory approvals.
VidiStar showed the VidiStar Accreditation Module for its echo reporting system. With VidiStar’s Accreditation Module, users will have a centralized repository for all of the accreditation needs including sample policies and procedures, data mining, flagged studies, quality assurance, surveys, etc. In addition, this new functionality allows users to configure their VidiStar reports to require users to fill out certain user-defined, accreditation report parameters prior to completing the report. VidiStar’s Accreditation Module can enhance echo, vascular and nuclear lab accreditation compliance as well as streamline the accreditation process.
As the largest global organization for cardiovascular ultrasound imaging, the American Society of Echocardiography (ASE) is the leader and advocate, setting practice standards and guidelines. Comprised of more than 16,000 physicians, sonographers, nurses, and scientists, ASE is a strong voice providing guidance, expertise and education to its members with a commitment to improving the practice of ultrasound and imaging of the heart and cardiovascular system for better patient outcomes.
For more information: www.asecho.org


 June 13, 2024
June 13, 2024