Fractional flow reserve (FFR) is considered the gold standard in determining the hemodynamic significance of coronary artery disease (CAD) lesions. It uses a pressure-derived index that measures coronary stenosis severity and can help determine whether to stent or not to stent. Despite guidelines encouraging the use of FFR, its use remains low because it requires use of vasodilator drugs, such as adenosine, which can increase procedure time, costs and patient discomfort. Addressing these concerns are two new technologies that recently completed clinical trials and may offer easier ways to perform FFR.
Data from the ADVISE II (use of instantaneous wave-free ratio (iFR) compared to FFR) and HeartFlowNXT (use of computed tomography (CT)-FFR compared to FFR) trials were presented at the 25th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium in October. Both technologies have stirred controversy over their ability to perform as well as FFR, and these trials show both these technologies may be viable alternatives.
Eliminating the Need for Adenosine
Instantaneous wave-free ratio (iFR) is a recently introduced, pressure-derived, adenosine-free index for assessment of coronary stenosis relevance. The ADVISE II trial used a hybrid iFR/FFR approach to determine the severity of CAD without the need for adenosine. The primary end-point was the percentage of stenoses properly classified in terms of hemodynamic severity by iFR, which it was able to do in 91.6 percent of the lesions tested.
“Overall, a hybrid iFR/FFR approach would avoid usage of adenosine in 69.5 percent of interrogated stenoses, whilst classifying correctly 94.2 percent of them in terms of hemodynamic severity,” said lead investigator Javier Escaned, M.D., Ph.D., an interventional cardiologist from Hospital Clinico San Carlos in Madrid, Spain.
He said eliminating the need for adenosine may encourage its usage by decreasing procedural costs and the time it take to perform FFR. “The simplicity of iFR can contribute to adoption of physiology in the cath lab and to faster interrogation of coronary vessels,” Escaned said.
The trial enrolled 797 patients in a prospective, observational, non-randomized, double-blind global multi-center registry study. The diagnostic performance of iFR was analyzed both as a dichotomous index and as part of a hybrid iFR/FFR strategy. iFR was used according to specific cutoffs (iFR zone, ?0.85 and ?0.94) with high positive and high negative predictive value for the detection of hemodynamically severe stenosis. FFR use was limited to the “adenosine zone,” for iFR values between 0.86 and 0.93. The primary endpoint was the percentage of stenosis properly classified in terms of hemodynamic severity (FFR value ? 0.80).
The final study population consisted of 690 stenoses and of these, 31 percent had an associated iFR value in the adenosine zone (0.86-0.93), while 69 percent had associated iFR values in the iFR zone. Within the iFR zone, the percentage of stenosis properly classified in terms of hemodynamic severity was 91.6 percent. Overall the hybrid iFR/FFR approach correctly classified 94.2 percent of coronary stenoses, without the need for adenosine administration in 65.1 percent of patients.
“The results of the ADVISE II study support the use of iFR to simplify physiological guidance of percutaneous coronary intervention,” Escaned said. “The promising results demonstrate the potential of boosting the benefits of ischemia-driven revascularization to a larger proportion of patients with coronary artery disease.”
Assessing FFR in the Next Big Stent Trial
The ADVISE II trial was funded by Volcano Corp., which is working to commercialize iFR. The technology will be further tested in the upcoming Volcano and Boston Scientific Corp. co-sponsored SYNTAX2 clinical study. The study will use a new clinical SYNTAX score to first identify prospective patients believed to benefit from stenting using modern-day drug-eluting stents and procedural guidance with FFR and intravascular ultrasound (IVUS). The goal is to show that delivering the most precise and modern-day stenting possible will allow more patients to be treated with catheter based procedures as an alternative to bypass surgery. SYNTAX2 will use live iFR/FFR hybrid measurements of the vessel to determine which patients and lesions are treated. Boston Scientific Synergy drug-eluting stents will then be placed with IVUS guidance, using both Volcano and Boston Scientific IVUS devices, to provide more accurate stent placement than has been demonstrated with angiography alone.
Promising Data on Noninvasive FFR
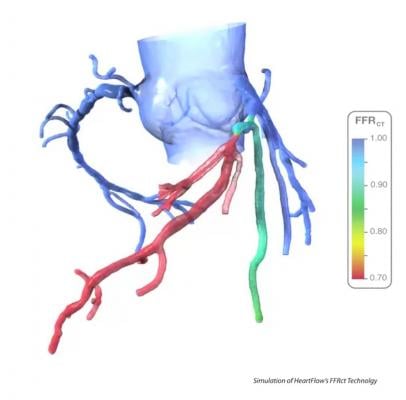
It may soon be possible to use a patient’s coronary computed tomography angiography (CTA) to noninvasively analyze the FFR for all the coronary vessels at once. The software developed by startup vendor HeartFlow can clearly identify culprit lesions or vessel segments with long-diffuse disease that cumulatively causes ischemia. The software creates a three-dimensional map of the coronary arteries showing locations of obstructions and CT-FFR values. The vessel segments can be color-coded based on their FFR values to quickly identify target lesion vessel segments.
The technology was tested in a head-to-head comparison with traditional FFR in the HeartFlowNXT trial, a blinded, prospective core lab adjudicated trial. The results were presented at TCT 2013 in October and demonstrated the CTA-based test accurately assesses coronary artery disease with results closely matching those of invasively measured FFR. The study also showed CT-FFR may offer better information than traditional FFR for potential revascularization treatment options, including angioplasty and coronary artery bypass surgery (CABG).
Current guidelines for the management of stable coronary artery disease (CAD) recommend noninvasive ischemia testing such as treadmill testing, single photon emission computed tomography (SPECT), stress echocardiography or cardiac magnetic resonance imaging (MRI) before invasive coronary angiography (ICA) or coronary revascularization is considered. However, the current noninvasive testing options such as treadmill testing, SPECT and stress echocardiography, correlate poorly to FFR, and thus selection of patients for invasive angiography and coronary revascularization is often inaccurate.
The primary study objective was to compare per patient diagnostic performance of CT-FFR compared to coronary CTA alone for the diagnosis of at least one hemodynamically significant stenosis, using direct measurement of FFR (?0.80) as the reference standard. The trial also measured per-vessel and per-patient diagnostic accuracy, sensitivity and specificity, as well as positive and negative predictive values of CT-FFR. The prospective study enrolled 254 patients scheduled to undergo non-emergent clinically indicated invasive angiography due to suspected coronary artery disease.
The CT-FFR data matched closely with invasively measured FFR. The area under the receiver operating characteristic curve for FFRCT (?0.80) was 0.82 versus 0.63 for coronary CTA (lumen reduction ?50 percent) (p<0.0001) with invasive FFR as the reference standard. Per-patient sensitivity and specificity were 86 percent and 79 percent for CT-FFR versus 94 percent and 34 percent for coronary CTA, and 91 percent and 51 percent for invasive coronary angiography (lumen reduction >50 percent).
“Fractional flow reserve noninvasively calculated from coronary CTA data sets matches closely with invasively measured FFR and allows for identification of patients with hemodynamically significant coronary lesions with good accuracy. When compared to both coronary CTA and ICA, FFR-CT led to a significant reduction in the proportion of false-positive results,” said lead investigator Bjarne L. Nørgaard, M.D., Ph.D., from Aarhaus University Hospital in Denmark. “The addition of FFRCT to coronary CTA allows for a comprehensive anatomic and functional assessment of coronary artery disease.”
Limits of the Current Technology
CT-FFR may one day eliminate the need to refer patients for diagnostic catheter angiographies. However, today, users of the HeartFlow technology say it requires sending the CT digital imaging and communication in medicine (DICOM) dataset to the company in California. It then takes the supercomputing power of the HeartFlow computer system about a day to process the data and render a report. While this may eliminate CT-FFR's use for acute chest pain patients, it offers an option for patients considering elective PCI. Coronary CT experts say computing speed and power doubles every couple years, which will eventually enable CT-FFR to be processed onsite in minutes, instead of days or hours.