
For chronic mitral valve regurgitation (MR) from degenerative disease, conventional open surgery provides a durable repair with a perioperative mortality rate that approaches <1 percent in experienced centers.[1,2] However, for functional MR, the results of open repair are significantly worse, with higher operative risk and lower long-term survival.[3,4] In most cases, functional MR results from ventricular remodeling associated with ischemic heart disease. Older age, renal failure, cerebrovascular disease and a history of previous cardiac surgery are common in this high-risk patient population and are the major contributors to poor outcomes. Perioperative mortality, by some analyses, may be as high as 17 percent.[5] This is reflected in the American Heart Association/American College of Cardiology (AHA/ACC) guidelines, which place severe MR as a class IIb indication for surgery in patients with functional MR.[6]
Many patients with concomitant pathology such as porcelain aorta, previous coronary artery bypass surgery or severely decreased left ventricular ejection fraction may have prohibitive risk for open surgery. Data examining the management and referral patterns of those with severe MR suggest that elderly patients with poor left ventricular function or significant comorbid disease are unlikely to be referred for mitral valve surgery.[7] A European analysis found that only 51 percent of patients with severe, symptomatic MR were taken for surgery.[8] These findings suggest there is a large population of patients with severe MR who are undertreated. They may have potential for longer, more productive lives and improved functional status if their MR could be addressed with a less invasive approach.
With the goal of treating patients at prohibitive risk for open heart surgery with effective percutaneous techniques, novel mitral valve repair devices have recently been developed. It is hoped that these promising new technologies can successfully repair diseased mitral valves to provide improved survival and quality of life while minimizing the risk of intervention.
Devices in Development
Several transcatheter devices, which are based on open surgical repair strategies, are presently under investigation. Open mitral valve repair is approached with a segmental analysis of the valve, which permits a targeted repair strategy. In the operating room, pathology such as leaflet prolapse can be addressed with leaflet suture-repair techniques, while annular dilation causing central regurgitation can be addressed with a downsizing ring, which shrinks the annulus and facilitates leaflet apposition. Recently, percutaneous devices, none of which are commercially available in the United States, have been developed to emulate the strategies of open repair. These devices can be categorized according to the pathology they are intended to address. Devices designed to address annular dilation can be percutaneously delivered and positioned directly on the annulus or in the coronary sinus to “cinch” the surrounding tissue and decrease annular size. The Carillon mitral contour system offered by Cardiac Dimensions is the only one in clinical use, having recently received CE mark approval in Europe.
To address leaflet pathology, innovative ablation and plication devices have been developed. Most promising among these is Abbott Vascular’s MitraClip system, which received CE mark approval in 2008, and is at the clinical trial stage of development in the United States.
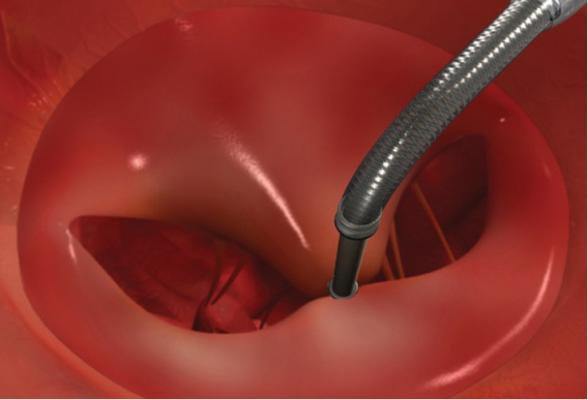
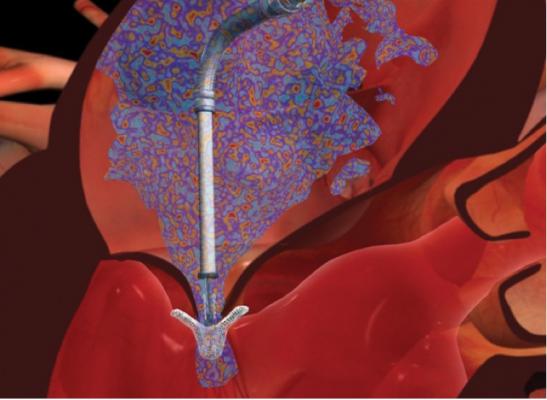
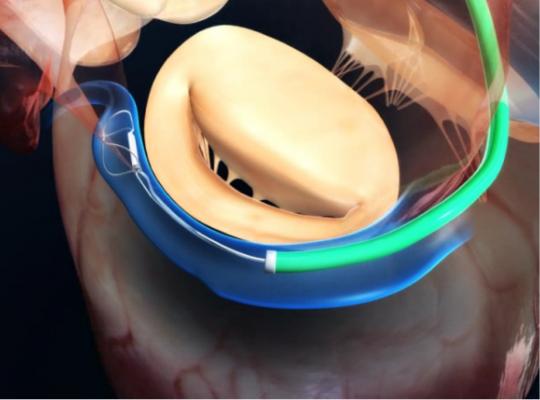
This device is based on the open Alfieri, or edge-to-edge mitral valve repair technique, which uses a stitch to approximate the middle portions of the anterior and posterior leaflets to create a double orifice valve.[10] This technique restores valve competence by facilitating leaflet coaptation and eliminating excessive leaflet motion. The device is deployed by transfemoral venous access using a catheter delivery device advanced across the interatrial septum to the mitral valve orifice. Using fluoroscopy and echo guidance, one or more clips are applied to grasp and approximate the free edges of the anterior and posterior leaflets. Clinical results have demonstrated safety and efficacy of the device, but suggest applicability may be limited to a subset of patients with functional MR and valve anatomy favorable for clip deployment.
Work on a percutaneously delivered bioprosthetic mitral valve may perhaps hold the greatest promise for the future. With the potential to treat both degenerative and functional mitral valve disease, transcatheter mitral valve replacement may eventually provide a percutaneous option for the broadest patient population. Although devices are currently under investigation, the lack of a rigid mitral annulus for device fixation presents a challenge for device engineers, which is yet to be overcome.
Percutaneous Mitral Repair: Equipment and Technical Support
Although minimally invasive, deployment of a MitraClip device requires a significant number of skilled personnel and highly specialized equipment. The ideal team involves the expertise of an interventional cardiologist, anaesthesiologist, echocardiographer, surgeon and nursing team. A hybrid operating room or cath lab is needed to acquire high-quality fluoroscopic image guidance of catheter placement and to maneuver the device across the interatrial septum using a trans-septal puncture. Access is obtained via the femoral vein for device delivery and a pigtail is placed through the contralateral femoral artery into the ascending aorta. The radial artery and internal jugular vein is cannulated for hemodynamic monitoring. After advancing the device into the left atrium, further manipulation is achieved with a proprietary steerable guide catheter and dilator assembly delivery system, which is controlled with a complicated release and steering mechanism.
The precise movements required for successful deployment necessitate 2-D transesophageal echo (TEE) guidance that allows the clip to be positioned directly above the regurgitant jet. When the clip is closed, echo assessment is essential to guide further clip positioning or placement of additional clips, depending on the degree of residual MR. Three-dimensional echo is strongly advised to appreciate the true geometry of the valve and to readily identify complications or imprecise clip deployment.
The COAPT Trial
The EVEREST II trial examined the safety and efficacy of the MitraClip system, demonstrating a successful reduction in MR resulting in favorable reverse ventricular remodeling and improved clinical symptoms.[11,12] Data from a cohort of patients with high surgical risk who underwent the MitraClip procedure were compared to results in non-high risk patients who underwent conventional mitral valve surgery. This cohort had shorter anaesthesia duration, shorter intensive care unit (ICU) stay and was discharged earlier than the non-high risk patients after conventional surgery. Recently, in the EVEREST II high-risk study, a cohort of patients with an estimated surgical mortality of ?12 percent underwent the MitraClip procedure. These results showed favorable results for safety and effectiveness in this high-risk patient population.[12]
Following from this, a parallel comparison between high surgical risk populations is being undertaken with multicenter enrollment of the COAPT trial. This prospective, randomized controlled trial will evaluate the safety and effectiveness of the MitraClip system when used for the treatment of functional MR in patients who are at extremely high risk for conventional open surgery. Patients deemed eligible will be randomized in a 1:1 fashion to treatment with the MitraClip system or to a control group that will not undergo the procedure. Those eligible for enrollment must have severe, symptomatic MR and be determined inoperable by a cardiac surgeon and an eligibility committee. They must also have valvular anatomy favorable for MitraClip deployment with a primary regurgitant jet that originates centrally. Safety will be assessed by a composite endpoint including death, stroke and other major events. Effectiveness endpoints will by assessed by the incidence of recurrent heart failure admissions, the severity of MR and heart failure symptoms.
The results of this study will help to define the benefit that may be gained by this transcatheter approach to mitral regurgitation. There is optimism that the MitraClip device will provide an option to increase the duration and quality of life of patients who are crippled by heart failure symptoms from mitral regurgitation and are too high risk for conventional open heart surgery.
Editor’s note: The authors, William Kent, M.D., MSc, Patrick McCarthy, M.D., Edwin McGee, M.D., and Chris Malaisrie, M.D., are all from the division of cardiac surgery, Bluhm Cardiovascular Institute, at Northwestern University in Chicago. Malaisrie serves as an editoral advisor for DAIC. Correspondence can be sent to [email protected].
References
5. Mehta RH, Eagle KA, Coombs LP, et al. “Influence of age on outcomes in patients undergoing mitral valve replacement.” Ann Thorac Surg 2002;74:1459-1467.
7. Treede H, Schirmer J, Rudolf V, et al. “A heart team’s perspective in interventional mitral valve repair: Percutaneous clip implantation as an important adjunct to a surgical mitral valve program for treatment of high-risk patients.” J Thorac Cardiovasc Surg 2012;143:78-84.
9. Fedak PW, McCarthy PM, Bonow RO. Evolving concepts and technologies in mitral valve repair. Circulation 2008:117:963-74.


 January 05, 2026
January 05, 2026 









