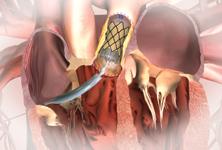
The FDA recently gave market clearance for Medtronic’s Melody Transcatheter Pulmonary Valve, the first transcatheter valve approved in the United States. The cardiology community expects this to be the first of many transcatheter valves to come, which are predicted to replace open-heart surgery with much less-invasive cath lab procedures.
“This is very important. It is a ground-shaking event for the congenital heart community,” said Tom Jones, M.D., director of Seattle Children’s Hospital’s cardiac catheterization laboratory and professor of pediatrics and medicine at the University of Washington School of Medicine, and an investigator with the Melody trial. “This is an important first step and I think it will open doors.”
Transcatheter valves offer shorter and easier procedures, less trauma to patients and faster recovery times. They also offer a nonsurgical alternative to patients who are not fit to undergo surgery.
“It’s going to have a big impact, there is no question,” said John P. Cheatham, M.D., FAAP, FACC, FSCAI, director, cardiac catheterization and interventional therapy, co-director, The Heart Center, Nationwide Children’s Hospital, Columbus, Ohio, and an investigator in Melody clinical trial. “It will hopefully cut down on the number of surgical procedures.”
Patients with bad pulmonary valves usually face numerous open-heart surgeries. The valve needs to replaced every six to 10 years as the valve wears out. Over time, Dr. Jones said the repeated surgeries do take a toll on the patients, leading to increased morbidity and mortality over time. That fact often delays needed surgery for long periods.
“We often wait longer than we would otherwise like because it has a cumulative effect on the patient over their lifetime,” Dr. Jones said.
The Melody can be used to defer these surgeries and reduce the number needed over a patient’s life. It helps restore valve function and effective blood flow to the lungs.
“It is a major breakthrough to restore the hemodynamics that would be expected after heart surgery,” Dr. Cheatham said.
The Melody trial included 150 patients treated at five sites. Just under 30 patients were treated at Nationwide Children’s Hospital. Dr. Cheatham said all of the patients who received the Melody at his hospital said they could do more than before the surgery. The change was noticeable the day after the implantation. In one case, he said a mother was able to keep up with her small child, where two days before she could not.
“It’s a pretty compelling story and it has definitely improved the lives of the patients treated with the Melody,” Dr. Cheatham said. “Every one of the patients had improved function and did not need to recover from the surgery first… The fact that they can go home the next day and not have another ‘zipper’ on their chest is also a big plus.”
Overall, Dr. Jones said he is also very impressed with the outcomes. “The recovery time is remarkably reduced to a few hours or days, as opposed to weeks,” he said. “How important is this to our patients? It’s monumental. It’s a real game changer.”
There is a question of how long the Melody will help delay surgery. So far, U.S. trials only have follow-ups up to three years, but durability of the valve has been shown out to five years in European studies.
“The function of the valve leaflets have far exceeded our original expectations,” Dr. Jones said. “We are seeing outstanding results with no more than mild regurgitation in the three-year follow-ups.”
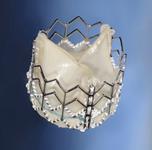
The device uses a balloon-expandable platinum iridium stent scaffold holding a bovine jugular vein conduit and tricuspid valve. Dr. Jones said the positive outcomes might, in part, be due to the metal stent structure helping to maintain the shape of the valve.
However, Dr. Jones said the stents in the pulmonary outflow track have a tendency to fatigue and fracture. He saw this happen during the Melody trial, however, most stent fractures were well tolerated without any significant change in the function of the valve. In a minority of cases when stent fracture led to deterioration in valve function, the problem could be resolved by implanting another Melody valve inside of the old one.
Dr. Cheatham helped design the stent used in the Melody. Percutaneous valves are a new technology and as more experience is gained, he said improvements will be made. He said Melody’s stent frame sits under the breast bone and is prone to fatigue. “We don’t have perfect stent materials yet,” Dr. Cheatham said.
Development of the Melody
Melody was the first transcatheter valve to receive regulatory approval anywhere in the world, when it received the CE mark clearance in Europe in October 2006. To date, more than 1,100 patients worldwide have received a Melody valve. Its FDA approval came under a humanitarian device exemption (HDE), a special regulatory approval for treatments intended for fewer than 4,000 U.S. patients per year. HDEs are granted for medical devices that have demonstrated reasonable safety and probable benefit, but not clinical effectiveness. Investigators in the Melody trial said the device will likely benefit about 3,000 patients a year.
The valve is indicated for patients with conduits with sizes between 16-22 mm who already have a surgically implant valve that is failing. “The purpose of the Melody valve device was to preserve an already degraded surgical conduit,” Dr. Cheatham said. “It’s not meant to take the place of surgery, but act as an adjunct to help restore function.”
During placement of the valve, Dr. Jones uses a balloon to get an exact size of the conduit. Careful attention is also paid to the location of coronary vessels so they are not occluded when the valve’s stent structure is expanded. He said that is the most common reason why patients were excluded from the Melody FDA study.
Surgeon, Interventionalist Cooperation
Transcatheter valve replacement requires a team effort from both surgeons and interventional cardiologists to achieve the best outcomes, Dr. Cheatham said. Cooperation between these specialists has resulted in very good results at Nationwide Children’s. Turf battles will prevent the rapid proliferation of transcatheter valves. “If people maintained that attitude it would set us back a few years,” he said.
Nationwide Children’s opened its first hybrid cathlab/ORs in 2004 and has opened more since. The rooms allow the close collaboration of surgeons and interventionalists in the same room during a procedure, or a series of procedures. Dr. Cheatham said this teamwork on congenital heart repairs over the last several years made the transition easy when performing transcatheter valve repairs.
“It’s really necessary to do a lot of the transcatheter valve procedures with the hybrid suite with both the surgeon and interventional cardiologist working together,” Dr. Cheatham said.
Dr. Cheatham said today 50 percent of the visitors from other hospitals come to Nationwide Children’s to see its hybrid labs. Visitors incorporate the knowledge gained from Nationwide’s experience into the hybrid suites they are developing.
Raj Makkar, M.D., director, interventional cardiology and cardiac catherterization laboratory at Cedars-Sinai Medical Center, and an investigator in the PARTNER trial, spoke at TCT 2009 about the SAPIEN valve. He agreed transcatheter valves will change the way structural heart defects are treated. “I think the legacy of this trial (PARTNER) will go beyond valve replacement… it will lead to greater collaboration with surgeons and interventional cardiologists,” he said.




 February 06, 2026
February 06, 2026 









