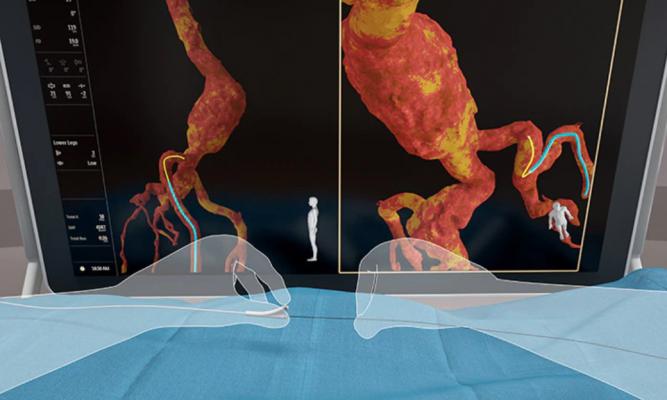
Philips Healthcare's new Fiber Optic RealShape (FORS) technology creates real-time 3-D imaging of anatomy and devices inside the body using light, rather than X-rays. FORS uses light traveling through hair-thin fiber optics inside special FORS enabled catheter and guidewires.
Philips is working on a prototype cath lab angiographic imaging system that might be able to replace the current X-ray fluoroscopy imaging systems used for interventional image guided procedures. As cath lab procedures become longer and more complex, there has been rising concern over how operators can reduce X-ray radiation exposure for themselves, their staff and their patients.
The new Fiber Optic RealShape (FORS) technology creates real-time 3-D imaging of anatomy and devices inside the body using light, rather than X-rays. FORS uses light traveling through hair-thin fiber optics inside special FORS enabled catheter and guidewires. The technology is based on the concept of measuring strain in the optical fibers, using light reflected from density fluctuations in these fibers. The location of the wires is then superimposed on 3-D anatomical imaging.
The FORS technology displays the full shape of devices in 3-D, in real-time and in distinctive colors, in multiple, user-controlled, unrestricted viewing angles and in context of the patient’s anatomy. It uses overlays from pre-operative 3-D anatomical data from computed tomography (CT), magnetic resonance imaging (MRI), or intra-operative rotational angiographic X-ray imaging.
Philips said it hopes to break through the current limitations of image guided therapy so clinicians can reduce their dependency on radiation-emitting fluoroscopy, while being able to see devices and anatomy more clearly inside patients during procedures. The FORS technology enables real-time 3-D visualization of devices inside the body without the need for fluoroscopy.
Philips hopes to move beyond incremental innovation and instead develop this new concept as a breakthrough imaging technology. If successful, FORS would be enable a radiation-free environment.
First-in-Human Use of the FORS Cath Lab Imaging Technology
The technology now has European CE mark clearance and its being used at University Medical Center Utrecht (UMC) Ulrecht in The Netherlands, and at four other sites in Europe. Five U.S. hospitals hope to soon start using the system under and investigational device exemption in 2020.
UMC-Ultrech ran a first-in-human feasibility study using the new technology to guide endovascular aortic repair (EVAR) and illiac or SFA PTA peripheral angioplasty procedures. The study enrolled 21 patients between July and December 2019. The study data were first presented at the Leipzig Interventional Course (LINC) 2020 by Dr. Joost van Herwaarden, associate professor of medicine, Department of Vascular Surgery, UMC.
"X-ray has two major drawbacks," van Herwaarden said. "First, its harmful for the surgeon and the whole team, and the second thing is that X-ray will only give you 2-D information, while it is 3-D anatomy. So, this new Fiber Optic RealShape technology will provide us with imaging without using X-ray and also with 3-D information, so procedures might be quicker and easier. I think it has huge potential."
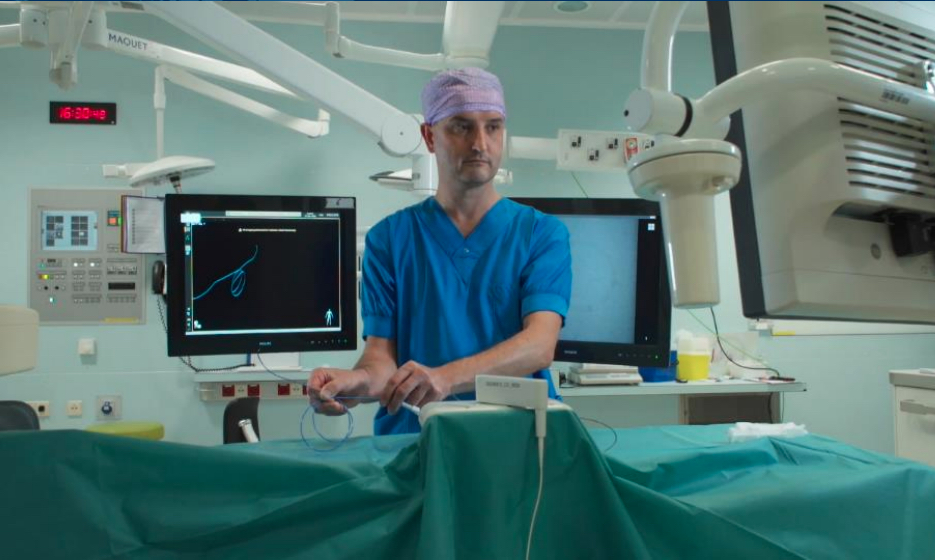
Dr. Joost van Herwaarden at University Medical Center Utrecht (UMC) Ulrecht in The Netherlands demonstrating Philips Healthcare's new FORS fiber optic light guided cath lab imaging system that eliminates the need for X-ray angiography during interventional cases.
In the study, the system was limited to the use of three FORS enabled devices. The operators were able to complete 60 out of 67 navigation tasks successfully using a FORS-enabled guidewire and/or a FORS-enabled catheter. Seven tasks were not completed successfully because different catheter shapes were needed. Overall, investigators rated the performance of FORS image guidance “better than standard guidance” in 16 (76%) cases and “on par with standard guidance” in five (24%) cases.
Key take aways van Herwaarden observed included the ability to navigate without the use of angiography, and the ability to use multiple unrestricted, viewing angles through huge caudo-cranial rotation of the anatomy that would be impossible to reach with a C-arm.
The FORS system also allows biplane visualization, where two simultaneous views from different angles can be projected ion the overhead screen to use as a roadmap to guide catheters. Van Herwaarden said FORS is also usable within deployed stent grafts.
Due to the great visibility of wire and catheter, which are highlighted on screen in bight, distinctive colors, digital subtraction angiography (DSA) can be used as a roadmap with the catheters overlaid.
"FORS appears to be a very promising, revolutionary new technology that has huge potential to improve endovascular procedures," van Herwaarden said.


 September 29, 2025
September 29, 2025 


