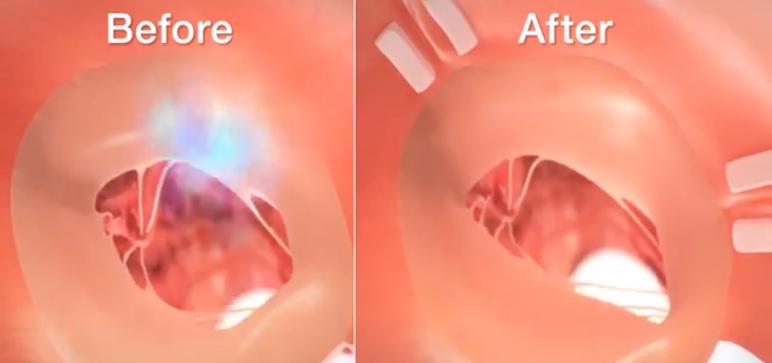
Image courtesy of Mitralign.
June 1, 2015 — Mitralign shared six-month data on its Mitralign Percutaneous Annuloplasty System (MPAS) for treatment of functional mitral regurgitation (FMR) at EuroPCR 2015 in Paris. The prospective, multi-center, single-arm study met its safety endpoint at 30 days and its performance endpoint at six months. In the clinical study, the MPAS demonstrated a statistically significant reduction in left ventricular diameter, significant reduction of both the A-P and S-L annular dimensions, and a significant improvement of the patient’s walking distance. The Mitralign Percutaneous Annuloplasty System is not approved for sale or distribution; however it is anticipated to receive CE marking in 2015.
“The data show statistically significant progress at six months, specifically in ventricular remodeling which is one cause of patient symptoms,” said Prof. Georg Nickenig of the University of Bonn. “These encouraging data further support the use of percutaneous valve repair to treat this challenging patient population.”
In his presentation, “Evaluation of the Mitralign Percutaneous Annuloplasty System for the treatment of FMR 6 month results” Nickenig showed that the system demonstrated a significant reduction in the anterior-posterior (A-P) and septal-lateral (S-L) dimensions of the annulus. Enhanced ventricular function was demonstrated by significant improvement in left ventricular end diastolic diameter and left ventricular end diastolic and systolic volumes. All patients were on optimal medical therapy and had an average ejection fraction of 33 percent.
In other EuroPCR presentations, Prof. J. Schofer, M.D., of the Medicare Center and Department for Percutaneous Interventions of Structural Heart Disease, Albertinen Heart Center, Hamburg, discussed his successful use of the MPAS to perform a direct transcatheter annuloplasty on special access patients with tricuspid regurgitation.
For more information: www.mitralign.com


 January 05, 2026
January 05, 2026 









