The current standard-of-care for catheter ablation of atrial fibrillation (AFib) patients includes up to six-hour long procedures with success rates that all electrophysiologists say can be improved. A possible break-through advance in ablation technology may help reduce procedure times by 80 percent, reduce damage to the heart and increase the effectiveness of ablation therapy.
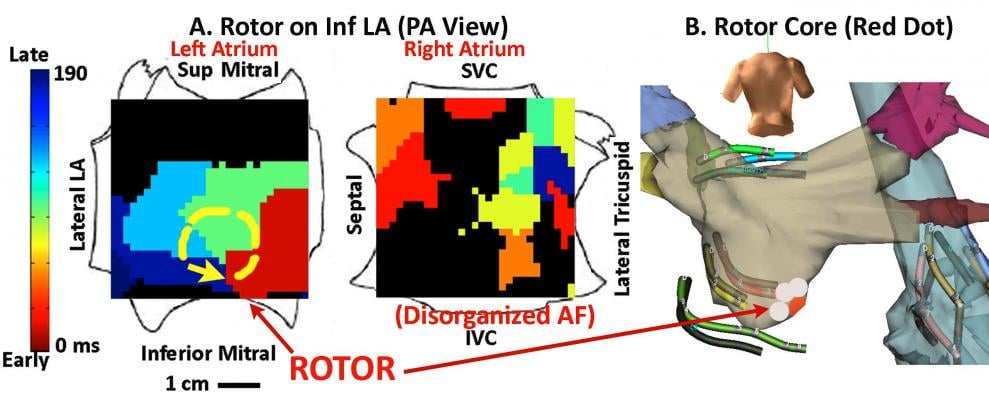
A new electromapping technique can better visualize the rotor (similar to the eye of a hurricane) around which electrical activity called initiating spirals rotate. Using this map, the electrophysiologist (EP) can position an ablation catheter on the rotor center and see immediately the results of the treatment. By using this targeted technique, in some cases a single ablation can terminate the AFib and restore the heart to normal rhythm. In conventional treatment, hours of ablation over a large area of the heart is needed to achieve a similar result.
“Incredible! This is going to turn the AFib world upside down,” said Warren "Sonny" Jackman, M.D., FHRS, George Lynn Cross research professor, senior scientific advisor, Heart Rhythm Institute, University of Oklahoma Health Sciences Center, Oklahoma City.
He said FIRM might be a big leap forward and be a paradigm shift in how AFib ablations are performed to achieve more efficiency and better patient outcomes.
The technique was presented at the Heart Rhythm Society (HRS) 2012 scientific sessions May 9 in Boston. Sanjiv M. Narayan, M.D., Ph.D., FHRS, associate professor of medicine in residence, co-director, EP fellowship training program, director, VA EP program, University of California San Diego School of Medicine, VA Medical Center, presented his research on targeted ablation at sources alone using focal impulse and rotor modulation (FIRM). He said the technique acutely terminates or slows human AFib.
Narayan said recent studies have shown that localized electrical rotors and focal beats may sustain human AFib. His team hypothesized that brief ablation (?10 min) of these sources could terminate AFib without pulmonary vein ablation.
In 51 AFib patients (63±10 years; left atrial, LA size 49±8 mm; 41 persistent), they recorded AFib using a 64-pole basket mapping catheters in both atria. Wave similarity and Hilbert analyses were used to detect AFib rotors or focal beats. This was visualized on a flattened, 2-D, “panoramic” electromap showing a cine loop of the electrical signal motion. This allows much easier visualization of rotors. Current technology requires EPs to look at numerous individual points on an electrogram and reconstruct the electrical motion in their minds. However, the FIRM map takes the guesswork out of visualization and more clearly shows the location of a rotor.
“You look at the electrograms and you cannot reconstruct this in your head,” Jackman said. “Based on an electrogram I could never have done that.”
Narayan’s team used FIRM ablation applied for less than 10 minutes/source to terminate or slow the AFib prior to pulmonary vein isolation. Patient-specific FIRM maps revealed rotors or focal beats in 50 of the 51 patients. The acute endpoint was achieved in 45 of 51 patients (an 88 percent success rate) with no other ablation. AFib terminated in 29 patients (57 percent) in 4.2 minutes. In 16 patients, FIRM slowed AF by 24±10 ms (15±3 percent).
Narayan said ablation at only the center of the rotor using FIRM terminated AFib to sinus rhythm in less than one minute. He explained computational mapping reveals rotors and focal beat drivers in nearly all persistent and paroxysmal AFib patients, where very brief FIRM ablation rapidly terminated or substantially slowed AFib. While the findings are very promising, we said more data is needed to determine how FIRM ablation may optimize procedural time and outcomes from AFib ablation.
The technique does not always work, Narayan said. He said the biggest predictor of failure seems to be if the basket is too big to get into the pulmonary vein, or are not seated properly in the atria, which means some areas are not mapped, giving an incomplete FIRM map. Incomplete seating in the atria can usually been seen under angiography, where the arms of the basket are not deformed, because they are not contacting the walls of the heart.
Narayan received a standing ovation at the conclusion of his presentation and for 40 minutes following the session was surrounded by doctors who wanted to learn more about the FIRM technique.


 January 05, 2026
January 05, 2026 









