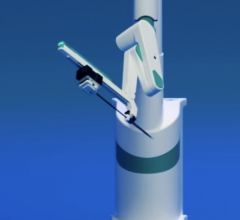
August 21, 2013 — The idea that surgery to relieve the pressure caused by hemorrhaging in the brain is a perfect job for a robotic system is the basic premise of a new image-guided surgical system under development at Vanderbilt University. It employs steerable needles about the size of those used for biopsies to penetrate the brain with minimal damage and suction away the blood clot that has formed.
The system is described in an article accepted for publication in the journal IEEE Transactions on Biomedical Engineering. It is the product of an ongoing collaboration between a team of engineers and physicians headed by Robert J. Webster III, assistant professor, and Kyle Weaver, assistant professor of neurological surgery.
For the last four years, Webster’s team has been developing a steerable needle system for “transnasal” surgery: operations to remove tumors in the pituitary gland and at the skull base that traditionally involve cutting large openings in a patient’s skull and/or face. Studies have shown that using an endoscope to go through the nasal cavity is less traumatic, but the procedure is so difficult that only a handful of surgeons have mastered it.
Webster’s design, which he calls an active cannula, consists of a series of thin, nested tubes. Each tube has a different intrinsic curvature. By precisely rotating, extending and retracting these tubes, an operator can steer the tip in different directions, allowing it to follow a curving path through the body. The single needle system required for removing brain clots was actually much simpler than the multi-needle transnasal system.
The brain-clot system only needs two tubes: a straight outer tube and a curved inner tube. Both are less than 1/20th of an inch in diameter. When a computed tomography (CT) scan has determined the location of the blood clot, the surgeon determines the best point on the skull and the proper insertion angle for the probe. The angle is dialed into a fixture, called a trajectory stem, which is attached to the skull immediately above a small hole that has been drilled to enable the needle to pass into the patient’s brain.
The surgeon positions the robot so it can insert the straight outer tube through the trajectory stem and into the brain. He also selects the small inner tube with the curvature that best matches the size and shape of the clot, attaches a suction pump to its external end and places it in the outer tube.
Guided by the CT scan, the robot inserts the outer tube into the brain until it reaches the outer surface of the clot. Then it extends the curved, inner tube into the clot’s interior. The pump is turned on and the tube begins acting like a tiny vacuum cleaner, sucking out the material. The robot moves the tip around the interior of the clot, controlling its motion by rotating, extending and retracting the tubes. According to the feasibility studies the researchers have performed, the robot can remove up to 92 percent of simulated blood clots.
“The trickiest part of the operation comes after you have removed a substantial amount of the clot. External pressure can cause the edges of the clot to partially collapse making it difficult to keep track of the clot’s boundaries,” said Webster.
The goal of a future project is to add ultrasound imaging combined with a computer model of how brain tissue deforms to ensure that all of the desired clot material can be removed safely and effectively.
For more information: www.vanderbilt.edu


 January 27, 2026
January 27, 2026