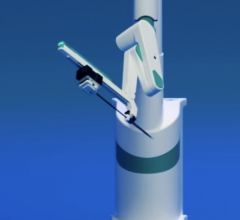
May 17, 2013 — The Journal of American College of Cardiology has published the results from Corindus Vascular Robotics’ CorPath PRECISE (Percutaneous Robotic-Enhanced Coronary Intervention) study in the April 2013 issue. Results of the trial demonstrate the vascular robotics CorPath System is safe and feasible for patients, with significantly lower harmful radiation exposure to the operator.
The study was a prospective, single-arm, multicenter, open-label, non-randomized study, enrolling 164 patients at nine clinical trial sites.
The primary endpoint of clinical procedural success was achieved in 97.6 percent of patients, and device technical success was achieved in 98.8 percent. Additionally, a secondary endpoint of the trial was the reduction in radiation exposure to the physician. Researchers determined the CorPath System significantly lowered radiation exposure by 95.2 percent compared to levels found when a physician performs the procedure at the patient’s bedside. Although operator comfort was not measured in the study, researchers noted the ability to perform procedures in a seated position without a heavy lead apron provides relief for back discomfort and allows the operator to focus solely on the patient.
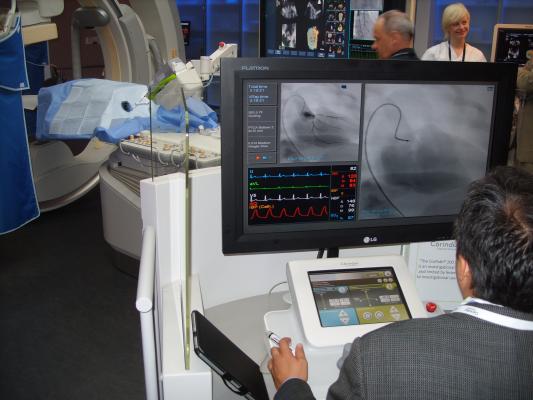
Traditionally, angioplasty procedures are performed manually, where an interventional cardiologist inserts a guidewire with a stent or balloon attached to physically open an artery blockage and help improve blood flow. While angioplasty procedures remain one of the most frequently performed procedures in the United States with nearly one million cases annually, the procedure has remained largely unchanged for decades. A CorPath robotic angioplasty allows interventional cardiologists to perform the procedure remotely, away from the patient bedside. Seated in a radiation-protected cockpit, the physician uses a joystick to robotically advance catheters, angioplasty balloons and stents to clear the blockage and restore blood flow. Additionally, the technology provides interventional cardiologists with the ability to accurately measure anatomy and precisely control stents.
“At Sanford Health, we implemented two CorPath Systems in December, and we have already performed 34 procedures. Using a joystick, I am able to advance the catheter millimeter-by-millimeter through the artery and achieve enhanced control when placing the stent,” said Tom Stys, M.D., FACC, FSCAI, medical director at Sanford Heart Hospital and Cardiovascular Services, Sioux Falls, South Dakota. “With an increasing amount of complex procedures being performed, we feel it is essential to invest in new technology that will help physicians provide enhanced patient care.”
For more information: www.corindus.com


 January 27, 2026
January 27, 2026