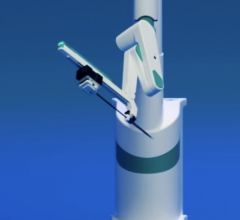
The Corindus CorPath 200 robotic-assisted PCI system allows operators precise remote control of catheters from a lead-shielded booth.
Interest has exploded over the past year in robotic cath lab guidance technology since Corindus began its U.S. Food and Drug Administration (FDA) pivotal trial. The Corindus CorPath 200 robotic-assisted percutaneous coronary intervention (PCI) system has been designed to provide precise, robotic-assisted placement of coronary guide wires and stent/balloon catheters in PCI procedures. The system allows operators to sit in a lead-lined booth to manipulate catheters by joystick, rather than wearing lead aprons and standing in or near the X-ray field.
Robotic systems, especially Corindus, were in the limelight at the 2011 Transcatheter Cardiovascular Therapeutics (TCT) meeting in November. A very positive review of the Corindus CorPath was presented at a packed breakfast session by investigators involved with the CorPath PRECISE trial. The FDA pivotal trial began in early 2010 and will enlist 175 patients across the United States. The results of this study will be the basis for a pre-market clearance 510(k) application to the FDA. Corindus hopes for final FDA review in the next 12-18 months.
Drinking the Kool-Aid
It is rare that a first-generation medical device is tested for the first time in patients and works exactly as planned. All of the speakers at the TCT program have “drunk the Kool-Aid,” so to speak, and praised the system’s capabilities with no reservations. All agreed it worked much better than anticipated. They all agreed robotic systems will likely be the future of cath labs.
“It’s like the first time you put on reading glasses and all of sudden you can see clearly,” said co-principal investigator Giora Weisz, M.D., co-director of clinical services at the Center for Interventional Vascular Therapy at New York Presbyterian Hospital/Columbia University Medical Center.
“I looked at this when it first came out of Israel and said, ‘we don’t really need this – this is a science project,’” said Peter Fitzgerald, M.D., director of the Center for Cardiovascular Technology at Stanford University Medical School, Calif. But, he, too, was enamored with its capabilities. “You just need to sit down in it and try it,” Fitzgerald said.
“This device really does sell itself once you get someone to sit down and use it,” said co-principal investigator Joseph P. Carrozza, M.D., chief of cardiovascular medicine at St. Elizabeth’s Medical Center, Boston. “This allows you to use any stent or any wire off the shelf. The system just allows you to use these devices better.”
“This first-generation device works amazingly well,” said Christopher Metzger, M.D., FACC, director of cardiac and peripheral vascular cath labs, Holston Valley Medical Center, Kingsport, Tenn. “I can’t tell you how much of a surprise it was that the robot works as well as it does.”
Ron Caputo, M.D., FACC, FSCAI, director of cardiac services and cardiology research at St. Joseph’s Hospital Health Center in Syracuse, N.Y., agreed. “Things have gone a lot better than we expected. You always expect the worst whenever you begin testing a new technology.”
“For the very first time in my career, I was able to perform a PCI procedure without wearing a lead apron,” said Jeffrey W. Moses, M.D., investigator of the CorPath PRECISE trial, director of interventional cardiology at New York Presbyterian Hospital, director of the Center for Interventional Vascular Therapy at New York Presbyterian Hospital/Columbia University Medical Center. “The CorPath System’s radiation-shielded cockpit provided an optimal view of the angiography screen and allowed me to easily manipulate the guide wire and accurately place the stent. I was impressed — this trial has the potential to significantly impact how we care for patients in the cath lab.”
Precision, Stability and Control
Moses said he was very skeptical about this and other robotic systems, because of the general feeling that a machine cannot replace the tactile skills of an experienced operator. “I rejected an article about a robotic system for Circulation 10 years ago. I thought it was like an erector set,” he said.
However, he said the navigation is more efficient and intuitive than he expected once he began using it in the PRECISE trial. He was also surprised at the very precise stent deployment and the system’s ability to stabilize the guidewire.
He said the system offers very good precision, stability and control, which may lead to better patient outcomes and shorter procedures. The system also offers excellent wire support and control to perform complex bifurcation stenting procedures.
“You have very discreet control – get accurate length measurement and precise device placement,” Moses explained. “You control fluoro, contrast media and balloon inflation.”
Caputo said there is a high rate of longitudinal geographic miss with manual stent placement, as much as 47.6 percent, according to a recent study he cited. However, this issue is eliminated with robotics because of its precision. This may lead to use of fewer stents and less contrast, which he said will reduce costs. He estimates operators can save one stent for about every eight cases. In addition, the increased precision and navigation reduced X-ray time and the use of contrast. Caputo said about $25 can be saved for every 100 cc of contrast conserved.
“I think there is potential to reduce radiation exposure to the patients,” Caputo said.
In addition, he likes the system’s 1 mm robotically assisted movements. “If you think you can move a device in 1 mm increments, you can’t, but the robot can,” he said.
While Metzger said he is comfortable with the robot in most cases, he still has reservations about using it with atherectomy procedures.
All these operators said the system enabled easier catheter navigation because of more precise catheter manipulation and prevention of wire “slippage.” Easier vessel navigation also means less radiation for patients and operators.
“It will essentially allow the operator to do what we have done since 1977 – except without the radiation exposure and with more precision,” Carrozza said.
Prolonging Interventionalists’ Careers
All robotic systems remove the operator from the X-ray field. Dose measurements in the PRECISE trial showed a massive cut in physician radiation exposure.
“We measured radiation exposure and there was a 97 percent reduction in exposure, without wearing a lead apron,” Weisz said.
Weisz quoted a recent survey stating that nearly 38 percent of interventional cardiologists will get cataracts due to radiation exposure. He said 60 percent report chronic back pain due to the heavy lead aprons they wear in the cath lab.
“We all know colleagues who had to cut their careers short because of orthopedic problems. We also know there is no safe level for cumulative radiation dose,” Carrozza said.
CorPath allows operators to perform their work while sitting in a chair in a lead-shielded booth without the need for the heavy aprons. “This will help enhance and prolong our careers,” Metzger said.
More Complex Cases
The PRECISE trial limits cases to the most straightforward, easy interventions. However, all the operators involved said they believe the system will offer its greatest contributions in more complex cases where precision is a must. “I view this as a race horse you are trying to hold back, because we want to use it for more complex cases,” Carrozza said.
Metzger agreed, adding, “There is amazing potential for this with use in chronic total occlusions and structural heart cases.”
Moses said future cath labs will include these, integrated with much more image navigation, including co-registered fluoro and 3-D computed tomography (CT) datasets.
Philips Healthcare took notice of the technology and announced a partnership with Corindus in April 2011. Philips plans to integrate the CorPath with its interventional cardiology solutions. As part of the agreement, Philips acquired a minority stake in Corindus. The financial terms were not disclosed.
Other Interventional Robotic Systems
Stereotaxis introduced the first robotic system, the Niobe Remote Magnetic Navigation System, in 2005. The system is cleared in the United States for electrophysiology (EP) ablation, coronary, peripheral and neurovascular navigation. It uses two large electromagnets on either side of the patient table to guide catheters remotely during complex procedures.
In early 2011, Stereotaxis introduced Epoch, a new modular platform for faster, more efficient and dynamic magnetic catheter control. In Europe, Stereotaxis also offers Vdrive, a mechanical robotic platform for remote manipulation of a growing array of devices, such as a loop diagnostic catheter.
Hansen Medical, which has already commercialized a robotic EP catheter guidance system, is developing the Magellan robotic peripheral vascular interventional system. The system is 510(k) pending and is not yet available in the United States. Hansen hopes to gain FDA clearance by mid-2012. The company entered a joint development agreement with Philips to integrate its robotic systems into Philips cath lab/EP systems.
Hansen’s Sensei EP ablation robotic catheter system allows accurate 3-D control of ablation catheter movement. In a survey presented as a poster at the 2011 American Heart Association (AHA) meeting, users reported an overall complication rate of 4.7 percent. After an average followup of 15 months, the success rate (defined as freedom from atrial tachyarrhythmia off anti-arrhythmic drugs) of robotic procedures was slightly superior at 67 percent compared with that of manual procedures at 64 percent. The survey included 1,728 patients with AF who were treated with the Sensei system at 12 different sites. Cappato’s Worldwide Survey (2010) reporting on 16,309 AF patients was used for historical control of manual ablation results.
In late November 2011, Boston Scientific announced an exclusive European agreement with Catheter Robotics Inc. (CRI) to market the CRI Amigo system, a remote-controlled catheter system. The Amigo received the CE mark in March 2010. The system is compatible with some Boston Scientific and other commercially available catheters. It lets physicians remotely maneuver mapping and ablation catheters designed to treat common cardiac arrhythmias during electrophysiology procedures.



 January 27, 2026
January 27, 2026